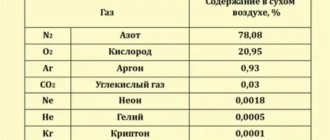
In meteorology, atmospheric pressure This is very important to consider when predicting and studying climate behavior. Clouds, cyclones, storms, winds, etc. They are largely caused by changes in atmospheric pressure.
However, atmospheric pressure is not something tangible, something that can be seen with the naked eye, so there are many people who understand the concept but don't actually know what it is.
Why don't we notice air pressure?
The force of universal gravity attracts everything to the Earth, including the atmosphere - the gaseous shell of the planet. At the same time, the upper layers of the atmosphere press on the lower ones. This is how atmospheric pressure arises. It's hard to believe, but a small table measuring 1x1 m is subject to pressure equal to the pressure produced by 10 cars. If this is really so, then why doesn’t the table break from such weight?
On every square centimeter of the surface of our body, air exerts a pressure approximately equal to that exerted by a load weighing 1 kg.
This does not happen because atmospheric pressure is transmitted in all directions, not just downward. Moreover, as far as you remember, according to Newton’s third law, the same force acts on this table, but only from below. And the atmospheric pressure is balanced by this force.
It is known that air presses on each of us with a force equal to the pressure of a load weighing more than 15 tons! That's the weight of three big trucks! Why don't our bodies collapse under atmospheric pressure? The fact is that the air inside each of our organs is also under pressure. And the internal air pressure balances the pressure acting on our body from the outside.
Cyclone or squall
On the contrary, when hot air rises, it reduces atmospheric pressure and causes instability. This is called a cyclone or storm . The wind always moves preferentially towards areas with lower atmospheric pressure. That is, whenever there is a storm in an area, the wind will be stronger because since there is less pressure in the area, the wind will go there.
Squall on the atmospheric pressure map
Another aspect to keep in mind is that cold and hot air do not mix immediately due to their density. When they are at the surface, the cold air pushes the warm air upward, causing a drop in pressure and instability. A storm then forms in which the area of contact between hot and cold air is called a front.
We cannot live without atmospheric pressure!
Strange but true: we really cannot live without atmospheric pressure! Even now, as you read this article, your body is using atmospheric pressure to move air in and out of your lungs. This suggests that thanks to atmospheric pressure we can breathe.
How do we breathe?
The diaphragm is the most important muscle when inhaling. It alternately contracts and relaxes, while the volume of the lungs and the internal pressure in them change. When the volume of the lungs increases, the pressure in them decreases, i.e. it becomes below atmospheric pressure, and air begins to enter the lungs. This is how inhalation occurs. As the pressure in the lungs increases, air escapes. This is an exhalation.
Diaphragm during breathing
How to measure atmospheric pressure?
In the middle of the 17th century. The outstanding Italian mathematician and physicist Evangelista Torricelli performed the following experiment. He took a glass tube about 1 m long, sealed at one end, and filled it with mercury. Then he turned the tube over and lowered it into a cup of mercury. As it turned out, a certain amount of mercury spilled into the cup, and the height of the mercury column remaining in the tube was 760 mm. At the same time, an airless space formed above the surface of the mercury in the tube.
Torricelli explained this phenomenon as follows. The surface of the mercury in the cup is exposed to atmospheric pressure, which is transferred to the tube. Because the mercury is in equilibrium, the atmospheric pressure is equal to the pressure created by the weight of the mercury column in the tube.
Pressure stage[edit | edit code]
The height to which one must rise or fall for the pressure to change by 1 hPa (hectopascal) is called the “pressure level”. The pressure stage is convenient to use when solving problems that do not require high accuracy, for example, to estimate pressure from a known height difference. Assuming that the atmosphere does not experience significant vertical acceleration (that is, it is in a quasi-static state), from the basic law of statics we obtain that the pressure level is equal to:
Change in atmospheric pressure
Torricelli also noticed that the level of the mercury column does not stay in one place, it changes: it either rises or falls. Based on his daily observations, the scientist concluded that if the pressure rises, the mercury column in the tube also rises, and vice versa. As a rule, fluctuations in atmospheric pressure are associated with changes in weather. If the pressure drops, rain and wind should be expected. If the pressure rises, the weather is expected to improve, and in winter it will also get colder.
What do experts advise to do at low atmospheric pressure?
The movement of mercury by more than one division in 3 hours is a reason for stress in the strong body of a healthy person. Each of us feels such fluctuations in the form of headaches, drowsiness, and fatigue. More than a third of people suffer from weather dependence to varying degrees of severity. In the zone of high sensitivity are populations with diseases of the cardiovascular, nervous and respiratory systems, and elderly people. How to help yourself if a dangerous cyclone is approaching?
There's not a lot of new advice here. It is believed that together they alleviate suffering and teach the correct way of life in case of weather vulnerability:
- See your doctor regularly. Consult, discuss, ask for advice in case your health worsens. Always have prescribed medications on hand.
- Buy a barometer. It is more productive to track the weather by the movement of the mercury column, rather than by knee pain. This way you will be able to anticipate the approaching cyclone.
- Keep an eye on the weather forecast. Forewarned is forearmed.
- On the eve of a weather change, get enough sleep and go to bed earlier than usual.
- Adjust your sleep schedule. Provide yourself with a full 8 hours of sleep, getting up and falling asleep at the same time. This has a powerful restorative effect.
- Meal schedule is equally important. Maintain a balanced diet. Potassium, magnesium and calcium are essential minerals. Ban on overeating.
- Take vitamins in a course in spring and autumn.
- Fresh air, walks outside - light and regular exercise strengthens the heart.
- Don't overexert yourself. Putting off household chores is not as dangerous as weakening the body before a cyclone.
- Accumulate favorable emotions. A depressed emotional background fuels the disease, so smile more often.
- Clothes made from synthetic threads and fur are harmful due to static current.
- Keep folk remedies for relieving symptoms in a list in a visible place. It’s hard to remember a recipe for herbal tea or a compress when your temples are aching.
- Office workers in high-rise buildings suffer more often from weather changes. Take time off if possible, or better yet, change jobs.
- A long cyclone means discomfort for several days. Is it possible to go to a quiet region? Forward.
- Prevention at least a day before the cyclone prepares and strengthens the body. Do not give up!
Don't forget to take vitamins to improve your health
Atmospheric pressure is a phenomenon that is absolutely independent of humans. Moreover, our body obeys it. What the optimal pressure should be for a person is determined by the region of residence. People with chronic diseases are especially susceptible to weather dependence.
Atmospheric air has a physical density, as a result of which it is attracted to the Earth and creates pressure. During the development of the planet, both the composition of the atmosphere and its atmospheric pressure changed. Living organisms were forced to adapt to the existing air pressure, changing their physiological characteristics.
- You need to ensure yourself proper rest and reduce your workload.
- Try to be outdoors for a short time.
- Avoid heavy foods, spicy seasonings and alcohol.
- You need to eat fractionally, in small portions.
- If you feel excessively nervous or have insomnia, use soothing decoctions or drops.
- Monitor your health, especially if you have any diseases related to the cardiovascular system.
- You need to reduce the load on your body and get more rest.
- Increase your diet with foods rich in vitamin E and potassium (nuts, dried fruits, seeds, dried apricots, bananas, carrots, beets, parsley, celery).
- Take a contrast shower, do light exercises, drink herbal teas.
- Spend as much time as possible outdoors.
It is believed that almost half of women who live in developed countries suffer from increased weather sensitivity. The number of weather-sensitive men is smaller - about one third. Weather-dependent people are most often susceptible to diseases of the heart and blood vessels, lungs, as well as endocrine diseases.
Sudden changes in temperature and frequent changes in weather in a short period of time often have negative consequences on health. It has already been noted that when the weather changes frequently, people call an ambulance much more often, which means they feel worse.
Despite the fact that there is no such diagnosis as “weather dependence,” doctors do not deny that the weather really does have an impact on our well-being. It is generally accepted that the weaker a person’s immunity and the more chronic diseases they have, the more sensitive the person is to weather changes. Why?
According to statistics, weather dependence is a hereditary trait in approximately 10% of cases. Most often it is inherited through the maternal line. 40% of cases of weather dependence occur due to serious vascular diseases. And the remaining 50% is age and the sores accumulated throughout life (from birth trauma to stomach ulcers or obesity).
The most common diseases leading to weather dependence are atherosclerosis, hypertension and hypotension, chronic respiratory diseases (angina, tonsillitis, pneumonia), as well as autoimmune diseases (for example, diabetes mellitus).
If weather dependence is observed in a child, then most likely it is a consequence of the mother’s difficult pregnancy, difficult childbirth, postmaturity or, conversely, prematurity.
As practice shows, most diseases acquired by a person throughout his life remain with him forever. Therefore, people with weather dependence can only monitor the weather reports and take appropriate measures to relieve symptoms.
Barometer
A device designed to measure atmospheric pressure is called a barometer.
Torricelli invented the mercury barometer, in which a column of mercury serves as a measure of atmospheric pressure. Such barometers are still in use today.
However, nowadays more modern liquid-free instruments, the so-called aneroid barometers, are more often used.
The height of mercury in the tube, equal to 760 mm, is taken as the standard of normal atmospheric pressure, which can be measured by the height of the mercury column (in mm). When we say that atmospheric pressure is, for example, 755 mm of mercury (mmHg), this means that the air produces the same pressure as a column of mercury 755 mmHg high. Art.
History[edit | edit code]
Traditionally, it was believed that suction pumps work because “nature is afraid of a vacuum.” But the Dutchman Isaac Beckman, in the theses of his doctoral dissertation, defended in 1618, argued: “Water raised by suction is not attracted by the force of emptiness, but is driven into an empty place by overlapping air” (Aqua suctu sublata non attrahitur vi vacui, sed ab aere incumbentein locum vacuum impellitur).
In 1630, the Genoese physicist Baliani wrote a letter to Galileo about an unsuccessful attempt to construct a siphon to lift water up a hill approximately 21 meters high. In another letter to Galileo (dated October 24, 1630), Baliani suggested that the rise of water in the pipe was due to air pressure.
The presence of atmospheric pressure led people to confusion in 1638, when the Duke of Tuscany's idea to decorate the gardens of Florence with fountains failed - the water did not rise above 10.3 meters. The search for the reasons for this and experiments with a heavier substance - mercury, undertaken by Evangelista Torricelli, led to the fact that in 1643 he proved that air has weight [5]. Together with V. Viviani, Torricelli conducted the first experiment in measuring atmospheric pressure, inventing the first mercury barometer - a glass tube in which there is no air. In such a tube, mercury rises to a height of about 760 mm.
How do we react to changes in atmospheric pressure?
Our body is adapted to living in conditions of normal atmospheric pressure, and, unfortunately, any changes in external pressure affect our well-being.
You already know that normal atmospheric pressure for a person is considered to be 760 mm Hg. Art. However, the barometer does not record such indicators so often. This is due to the fact that the pressure on the Earth's surface is unstable and uneven. The amount of atmospheric pressure depends on the time of day, season and various geographical conditions. As a rule, daily pressure fluctuations are no more than 4-5 mm. We don’t notice such a minor difference and tolerate it well.
For people living in the Peruvian Andes at an altitude of 4500 m above sea level, acclimatization begins in early childhood. Even their internal organs adapt to local conditions. Thus, the size of the chest of a mountain dweller is much larger than that of a person living on the plain.
Atmospheric pressure on ancient Earth was two times lower than today
Lava flows capture air bubbles and, when hardened, preserve samples of the atmosphere for researchers; and their job is to decipher these meteorological messages. Photo from deviantart.com
Archean volcanic basalt rocks, 2.74 billion years old, preserve traces of gas bubbles captured from the environment by liquid lava. An international team of geophysicists, based on the size of these traces, calculated the atmospheric pressure on the ancient planet. It turned out to be two times lower than the modern one. According to scientists, such low pressure is due to the small amount of nitrogen in the Archean atmosphere. The low density of the atmosphere means that the characteristics of important physical and chemical processes must be adjusted. In addition, it was previously believed that the heating of the planet was due to increased absorption of infrared radiation by the dense atmosphere. New data force us to reconsider this hypothesis. The most likely replacement is high concentrations of greenhouse gases, presumably methane.
It is difficult to imagine a topic more alluring, but also less accessible to study, than the beginning of earthly life. The main problem here is not a lack of ideas, but the rarity of reliable material evidence of those long-gone eras. We are talking about the Archean, that is, about a time approximately 3.8–2.7 billion years ago. Since then, little has survived the turbulent history of planetary change. All the more valuable are those solid grains of factual information on the basis of which one can build a building of testable hypotheses. A new block of such information was used by scientists from the University of Washington together with colleagues from the University of Western Australia and the Museum of Nature and Science in Denver (USA) to reconstruct the Earth's earliest atmosphere. Their findings force us to seriously reconsider, or at least think about, the currently accepted hypothetical portrait of the ancient Earth.
This team has been studying Archean sediments in the Pilbara region of Australia for several years. In this case, they worked with rocks from the Boongal Formation. The age of these deposits is estimated to be late Archean, that is, 2.75 billion years. This is quite an interesting age: the atmosphere of the planet during this period did not move too far from its state at the beginning of earthly life. At least, there were still 300 million years left before the start of the oxygen revolution.
In the Bungal formation there are volcanic layers, in some places, as the features of their structure show, formed in the coastal sea zone. For geologists, this means that the lava tongues solidified on the earth's surface, and not underground or under water on the ocean floor, and at zero altitude above sea level, and not on a kilometer-long volcanic crater. It was precisely these areas of ancient landscapes that scientists selected to solve the problem of measuring atmospheric pressure. With other unknown parameters—questionably reconstructed overlying layers of earth rocks, or the depth of the ocean, or altitude above sea level—the problem would be solved, at best, with a large tolerance, or rather, it would not be solved at all. But for the selected paleolandscapes these factors could be neglected.
The material basis for the reconstructions was traces of gas bubbles captured by lava flows from the atmosphere during solidification. Naturally, over billions of years, practically nothing remained of the atmosphere itself in these bubbles. They were replaced by elements of the parent rock and secondary minerals, turning into spots of a different color, composition and texture. But at the same time, their round shape remained unchanged. If the rock itself were deformed or for one reason or another experienced additional pressure, the bubbles would flatten and microcracks would appear. And since there is neither one nor the other, it means that the size of the bubble spots has not changed over the long history of rock transformations. Therefore, based on the size of the spots, it is possible to calculate the pressure at which they formed. The size of the bubbles on the lava surface is controlled only by atmospheric pressure, and as the depth of the lava flow increases, the pressure of the lava material itself is added to the atmospheric pressure. There are more bubbles at the surface and fewer at the bottom. Knowing the difference in the sizes of bubbles at different depths and the parameters of the volcanic material that determines the pressure in the flow, it is possible to estimate the atmospheric pressure. This method has already been successfully tested to measure atmospheric pressure at different altitudes above sea level for younger volcanic deposits in Turkey and China.
Traces of gas bubbles in basalt rock of the Bungal formation. Arrow
points to a trace with concentric circles confirming the gradual and later filling of the bubble.
The shape of the marks is round and cracks around the bubbles are not visible. The length of the scale bar is
1 cm. Photo from the discussed article in
Nature
So, here is the size of the bubbles in different layers of volcanic basalt, here is the thickness of the volcanic layers with bubbles, here is the density of molten basalt. From these data the pressure of the ancient atmosphere is easily calculated: 0.23±0.23 atm. It is not easy to assess the reliability of such low values. But the scientists referred to their previous conclusions (SM Som et al., 2012. Air density 2.7 billion years ago limited to less than twice modern levels by fossil raindrop imprints), which were made based on studying traces of ancient raindrops preserved in approximately those same Archean layers. In a certain romantic mood, one can imagine how, in the calm of wind, raindrops fall on the black ashes, covering it with pockmarks of wet holes, the air smells of ammonia freshness, the methane silence is broken by the shrill patter of drops. This ancient intaglio, sealed with layers of fine dust, forever preserved in the stone past the memory of that rain.
But dry physical calculations leave out the amazement at the natural miracle, taking into account only the depth of the holes from those raindrops. They can be measured, and from these measurements the speed of falling drops can be estimated, and knowing this speed, proceed to the density of the atmosphere. Raindrops gave pressure values of the order of 0.52–1.1 atm, while scientists saw the lower estimate of 0.52 atm as more likely, rather than the upper estimate of 1.1 atm. Taking into account previous and new data, a value of 0.5 atm was adopted for the Late Archean atmosphere. The low atmospheric pressure is explained by the significantly lower nitrogen content in it. In the absence of oxygen weathering of igneous rocks, its amount should be at least half that in the modern atmosphere. Presumably, nitrogen was present in the atmosphere in the form of ammonia and cyanide compounds.
What does such low atmospheric pressure provide for the reconstruction of other, indirect, conditions on the ancient Earth? It is known that at that time there was flowing, unfrozen water on the planet; there was no glaciation. With the low glow of the Sun - and it was then about 20% paler than today - some conditions should have ensured the conservation of heat. It was believed that such insulation could be provided by a dense atmosphere that absorbs infrared radiation and a high content of carbon dioxide, which provides a greenhouse effect. But if the atmosphere is deleted from this list, then only carbon dioxide remains. And its share in the atmosphere, according to available data, was not high enough to support proper heating of the planet. This means that the main role in this process belonged to other greenhouse gases, such as methane.
In addition, the low atmospheric pressure suggests that water boiled at a significantly lower temperature - 58°C. This means that the speeds and directions of chemical processes differed from modern ones. The rates of the photochemical reaction of fractionation of sulfur isotopes (see Mass-independent fractionation), which occurs under the influence of ultraviolet radiation, also differed. In all likelihood, new calculations of mass-independent fractionation with adjusted atmospheric parameters will be required. After all, a significant part of the reasoning about climatic conditions and life on the ancient planet is based on them.
Sources:
1) Sanjoy M. Som, Roger Buick, James W. Hagadorn, Tim S. Blake, John M. Perreault, Jelte P. Harnmeijer and David C. Catling.
Earth's air pressure 2.7 billion years ago constrained to less than half of modern levels // Nature Geoscience
.
Published online 09 May 2021. DOI: 10.1038/ngeo2713. 2) Sanjoy M. Som, David C. Catling, Jelte P. Harnmeijer, Peter M. Polivka, Roger Buick. Air density 2.7 billion years ago limited to less than twice modern levels by fossil raindrop imprints // Nature
. 2012. V. 484. P. 359–362. DOI: 10.1038/nature10890.
Elena Naimark
Pressure at altitude
You already know that the upper layers of the atmosphere exert pressure on the lower ones. This means that the air at the surface of the Earth is maximally compressed. However, the higher we rise above the Earth, the fewer layers of air become that compress the lower layers, and accordingly, the pressure decreases. It is precisely these pressure drops that we immediately feel on ourselves.
Remember: the higher the altitude, the lower the atmospheric pressure.
Geography 6th grade
summary of other presentations
“Mountains” 6th grade - Elements of mountains. Andes, Cordillera, Alps, Caucasus, Tien Shan. Problem conversation. Goals and objectives of the lesson. The highest mountains in Russia are the Caucasus. Mountains of sushi. The highest mountains in Europe. Answer the questions. Watching the film fragment “Mountains of Sushi”. Forces shaping the Earth's topography. Cordillera. Basic landforms of the Earth. How to describe the geographical position of mountains. New concepts of the topic.
“Natural areas and their inhabitants” - Penguins are a well-distinct group of birds with ancient origins. The penguin is the only bird that can swim but cannot fly. Giant kangaroo jumper. The homeland of chinchillas is South America. The main color of Grevy's zebra is white or white-yellow. Cat otter. Pronghorn. Koala. Sekuran fox. Chinchillas. Maltese lizard. Big red kangaroo. Giraffes live in the savannas of Africa.
"Waterfalls of the World" - The waterfall was originally discovered in 1910 by a Spanish explorer named Ernesto Sanchez La Cruz. Rivers, life-giving juices, Throw a waterfall into the abyss...... The legend of the origin of Niagara Falls. The height of the fall is so great that before reaching the ground, the water is sprayed into tiny particles and turns into fog. The most beautiful waterfalls in the world. It is believed that the first European to see the waterfall was David Livingston.
“Relief formation” - The action of external forces. Action of external forces of the Earth. Formation of the Earth's relief. The power of falling water. Accumulation of sedimentary material. Internal and external forces. Map task. Transformation of sediments into rock layers. Internal and external forces of the Earth. Mountains. The activity of internal and external forces of the Earth. Relief formation. Plant roots. The power of a tidal wave. Relief. The work of the wind changes. Cordillera Mountains, Andes.
“Game in Kazakhstan” - My Motherland - Kazakhstan. Bronze is an alloy of copper and gold. The Eneolithic is the time of the appearance of iron. Where and when were the remains of ancient man first discovered? A clan community is a permanent, stable group of relatives. Historical quiz. Tribal community. Pithecanthropus is one of the oldest people on Earth. Name the chronological framework of the Stone Age - Paleolithic. Chinaward. What was the name of the original group of people?
““Atmosphere of the Earth” 6th grade” - In which wind direction does precipitation most often occur? The following instruments are used to measure which weather elements. Calculate the average annual air temperature. Name the weather elements. Barometer. What is the name of the layer of the atmosphere where weather is formed? Describe the observed phenomenon and explain it. Where the bulk of atmospheric air is found. What is called saturated air. List climate-forming factors.
Why do we feel this
On earth, the air pressure in the ear tympanum is equal to normal atmospheric pressure. And when the plane gains altitude, the pressure decreases, and a pressure difference arises, i.e. our eardrum becomes depressed. This is why we feel stuffy in the ear.
The most familiar example is the “blocking” of ears on an airplane during takeoff. How to alleviate this condition? There are options:
- Open your mouth wide.
- Make several swallowing movements.
Geography
Why is atmospheric pressure low along the equator and high above the poles?
Near the equator, the air becomes very hot, expands and rises. Therefore, low pressure is formed. Around the poles, the air is heavy due to low temperatures. It goes down and the pressure gets high
More on the topic
Natural areas of the earth. What are the similarities and differences between equatorial rainforest and mixed forest?
How do living organisms differ from nonliving ones?
Complete the sentences with the names of the properties of air.
What is the Universe?
Why do we need geography and how will we study it? The textbook orients you in geography. Would you like to choose a different name? Which? Do you have your own symbol - a guideline in life?
Do you know what a compass is? Have you ever used it?
How are all parts of the hydrosphere connected to each other?
What makes up the world of living nature on Earth?
Which layer of the Earth is called the atmosphere?
Name which geographical science studies: processes occurring in the World Ocean; population of the Earth; processes occurring in the soils on which structures are erected; climates of the globe; composition and structure of the earth's crust; relief of the earth's surface; the influence of the characteristics of the territory on the health of the population.
If you liked the material and found it useful, share it with your friends!
About the site
On our website you will find many useful calculators, converters, tables, as well as reference materials on basic disciplines.
The easiest way to do calculations online is to use the right online tools. Use the search to find the right tool on our website.
calcsbox.com
The site uses LaTeX technology.
Therefore, to correctly display formulas and expressions
please wait until the page loads completely.
© 2021 All calculators online
Copying materials is prohibited
Source
Pressure changes in the mountains
In the mountains at an altitude of 2500-3000 m above sea level, the atmospheric pressure is much lower than at the foot. In such conditions, due to the difference in pressure inside the body and atmospheric pressure, our body is subjected to significant stress. Moreover, it is possible that signs of mountain sickness may appear: ear pain, difficulty breathing, nausea and weakness may occur.
In trained climbers and people permanently living in mountainous areas, such ailment is extremely rare. This is due to the fact that their body has already adapted to low blood pressure conditions.
At-risk groups
This group mainly includes people with chronic diseases and the elderly with age-related health changes. The risk of weather dependence increases in the presence of the following pathologies:
- Respiratory diseases (pulmonary hypertension, chronic obstructive pulmonary disease, bronchial asthma). Severe exacerbations occur.
- Damage to the central nervous system (stroke). There is a high risk of recurrent brain damage.
- Arterial hypertension or hypotension . A hypertensive crisis with the development of myocardial infarction and stroke is possible.
- Vascular diseases (atherosclerosis of the arteries). Atherosclerotic plaques can break away from the walls, causing thrombosis and thromboembolism.
Pressure underwater and underground
Representatives of some professions are forced to work in conditions of low air pressure. These are miners, divers and workers of caissons - special structures used to build bridges and other water structures. Descending into a deep shaft, miners experience increased atmospheric pressure. In very deep mines it can reach about 850 mm Hg. Art.
The pressure underwater is also much higher than atmospheric pressure. So, for example, when diving to a depth of about 100 m, the diver will be exposed to pressure that is approximately 10 times greater than atmospheric pressure!
Notes[edit | edit code]
Sources[edit | edit code]
Footnotes[edit | edit code]
- ↑ The formula assumes the temperature is the same at all altitudes. In fact, the temperature of the atmosphere changes with altitude according to a rather complex law. Nevertheless, the formula gives good results, and it can be used at altitudes of up to 50-100 kilometers. Thus, it is not difficult to determine that at the altitude of Elbrus - about 5.6 km - the pressure will drop by approximately half, and at an altitude of 22 km (the record height for the rise of a stratospheric balloon with people), the pressure will drop to 50 mm Hg. Art.
Difficulties of working as a diver
Diving to depth is only possible in special diving suits, and a rubber suit is used for diving to no more than 40 m. Working at great depths is possible only in a hard suit, which takes on all the water pressure
When a diver spends a long time in conditions of high water pressure, part of the air he breathes dissolves in the blood. In this case, the nitrogen contained in the air is not used by the body, but accumulates in the blood. As it rises to the surface, nitrogen is released in the form of bubbles that can clog blood vessels. To prevent these problems from occurring, the diver is lifted very slowly!
If a diver worked for an hour at a depth of 30 m, then reaching the surface is carried out within an hour, and if the diver spent the same hour at a depth of 60 m, then the ascent lasts 6 hours!
Share link