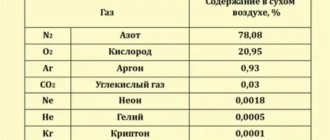
Atmospheric pressure refers to the pressure of the thickness of atmospheric air on the surface of the Earth and objects located on it. The degree of pressure corresponds to the weight of atmospheric air with a base of a certain area and configuration.
The main unit of measurement of atmospheric pressure in the SI system is Pascal (Pa). In addition to Pascals, other units of measurement are also used:
- Bar (1 Ba=100000 Pa);
- millimeter of mercury (1 mm Hg = 133.3 Pa);
- kilogram of force per square centimeter (1 kgf/cm2=98066 Pa);
- technical atmosphere (1 at = 98066 Pa).
The above units are used for technical purposes, with the exception of millimeters of mercury, which is used for weather forecasts.
The main instrument for measuring atmospheric pressure is the barometer. Devices are divided into two types - liquid and mechanical. The design of the first is based on flasks filled with mercury and immersed with the open end in a vessel with water. The water in the vessel transmits the pressure of the atmospheric air column to mercury. Its height acts as an indicator of pressure.
Mechanical barometers are more compact. The principle of their operation lies in the deformation of a metal plate under the influence of atmospheric pressure. The deforming plate presses on the spring, which, in turn, sets the arrow of the device in motion.
Introduction
It's raining outside the window today.
After the rain, the air temperature decreased, humidity increased and atmospheric pressure decreased. Atmospheric pressure is one of the main factors determining the state of weather and climate, so knowledge of atmospheric pressure is necessary in weather forecasting. The ability to measure atmospheric pressure is of great practical importance. And it can be measured with special barometer devices. In liquid barometers, as the weather changes, the liquid column decreases or increases. Knowledge about atmospheric pressure is necessary in medicine, in technological processes, in human life and in all living organisms. There is a direct connection between changes in atmospheric pressure and changes in weather. An increase or decrease in atmospheric pressure can be a sign of weather changes and affect a person’s well-being.
Description of three interrelated physical phenomena from everyday life:
- Relationship between weather and atmospheric pressure.
- Phenomena underlying the operation of instruments for measuring atmospheric pressure.
- Dependence of liquid pressure on the height of the liquid column in liquid barometers.
Relevance of the work
The relevance of the chosen topic is that at all times people, thanks to their observations of animal behavior, could predict weather changes, natural disasters, and avoid human casualties.
The influence of atmospheric pressure on our body is inevitable; sudden changes in atmospheric pressure affect a person’s well-being, and weather-dependent people especially suffer. Of course, we cannot reduce the influence of atmospheric pressure on human health, but we can help our own body. The ability to measure atmospheric pressure, knowledge of folk signs, and the use of homemade instruments can help to properly organize your day, distribute time between work and rest.
Purpose of the work: to find out what role atmospheric pressure plays in human daily life.
Tasks:
- Study the history of atmospheric pressure measurement.
- Determine whether there is a connection between weather and atmospheric pressure.
- Study the types of instruments designed to measure atmospheric pressure, made by man.
- Study the physical phenomena underlying the operation of instruments for measuring atmospheric pressure.
- Dependence of liquid pressure on the height of the liquid column in liquid barometers.
Research methods
- Literature analysis.
- Summarizing the information received.
- Observations.
Field of study: atmospheric pressure
Hypothesis : atmospheric pressure is important for humans.
Significance of the work : the material of this work can be used in lessons and in extracurricular activities, in the lives of my classmates, students of our school, and all lovers of nature research.
Work plan
I. Theoretical part (information collection):
- Review and analysis of literature.
- Internet resources.
II. Practical part:
- observations;
- collecting weather information.
III. Final part:
- Conclusions.
- Presentation of work.
Who is susceptible to weather dependence?
Often, dependence on the weather is associated with a person’s health status. For example, among those who do not have cardiovascular pathologies, about 5-10% are weather dependent. But among patients with hypertension, about 50% are meteodependent [3].
During temperature changes and other weather changes, symptoms may increase in people suffering from chronic diseases:
- spine and joints (arthrosis, arthritis);
- cardiovascular system (drops in blood pressure);
- respiratory system (asthma, chronic bronchitis);
- nervous system: dizziness, migraines, anxiety disorders;
Those who have had head injuries or fractures may also feel worse. Also at risk are people exposed to bad habits (smoking, alcohol), leading a sedentary lifestyle and pregnant women.
You can read the continuation of the article by following the link to RBC Style.
History of atmospheric pressure measurement
We live at the bottom of a huge ocean of air called the atmosphere. All changes that occur in the atmosphere certainly have an impact on a person, on his health, lifestyle, because... man is an integral part of nature. Each of the factors that determine the weather: atmospheric pressure, temperature, humidity, ozone and oxygen content in the air, radioactivity, magnetic storms, etc. has a direct or indirect effect on human well-being and health. Let's focus on atmospheric pressure.
Atmospheric pressure is the pressure of the atmosphere on all objects in it and the Earth's surface.
In 1640, the Grand Duke of Tuscany decided to build a fountain on the terrace of his palace and ordered water to be supplied from a nearby lake using a suction pump. The invited Florentine craftsmen said that this was impossible because the water had to be sucked up to a height of more than 32 feet (more than 10 meters). They could not explain why the water is not absorbed to such a height. The Duke asked the great Italian scientist Galileo Galilei to figure it out. Although the scientist was already old and sick and could not engage in experiments, he nevertheless suggested that the solution to the problem lay in the area of determining the weight of air and its pressure on the water surface of the lake. Galileo's student Evangelista Torricelli took up the task of resolving this issue. To test his teacher's hypothesis, he conducted his famous experiment. A glass tube 1 m long, sealed at one end, was completely filled with mercury, and tightly closing the open end of the tube, turned it over with this end into a cup with mercury. Some of the mercury poured out of the tube, some remained. An airless space formed above the mercury. The atmosphere presses on the mercury in the cup, the mercury in the tube also presses on the mercury in the cup, since equilibrium has been established, these pressures are equal. To calculate the pressure of mercury in a tube means to calculate the pressure of the atmosphere. If atmospheric pressure increases or decreases, the column of mercury in the tube increases or decreases accordingly. This is how the unit of measurement of atmospheric pressure appeared - mm. rt. Art. – millimeter of mercury. While observing the level of mercury in the tube, Torricelli noticed that the level was changing, which meant that it was not constant and depended on changes in the weather. If the pressure rises, the weather will be good: cold in winter, hot in summer. If the pressure drops sharply, it means that cloudiness and saturation of air with moisture are expected. A Torricelli tube with a ruler attached represents the first instrument for measuring atmospheric pressure - a mercury barometer. (Annex 1)
Mercury barometer
Other scientists also created barometers: Robert Hooke, Robert Boyle, Emil Marriott. Water barometers were designed by the French scientist Blaise Pascal and the German burgomaster of the city of Magdeburg, Otto von Guericke. The height of such a barometer was more than 10 meters.
To measure pressure, different units are used: mm of mercury, physical atmospheres, and in the SI system - Pascals.
Relationship between weather and atmospheric pressure
In Jules Verne's novel “The Fifteen-Year-Old Captain,” I was interested in the description of how to understand barometer readings.
“Captain Gul, a good meteorologist, taught him to understand the barometer readings. We will briefly tell you how to use this wonderful device.
- When, after a long period of good weather, the barometer begins to fall sharply and continuously, this is a sure sign of rain. However, if good weather lasts for a very long time, then the mercury column can drop for two or three days, and only after that any noticeable changes will occur in the atmosphere. In such cases, the more time passes between the start of the mercury fall and the start of rains, the longer the rainy weather will persist.
- On the contrary, if during a long period of rain the barometer begins to rise slowly but continuously, the onset of good weather can be confidently predicted. And good weather will remain the longer, the more time has passed between the beginning of the mercury rise and the first clear day.
- In both cases, a change in weather that occurs immediately after the rise or fall of the mercury column persists for a very short time.
- If the barometer rises slowly but continuously for two or three days or longer, this portends good weather, even if it has been raining non-stop all these days, and vice versa. But if the barometer rises slowly on rainy days, and immediately begins to fall when good weather comes, the good weather will not last long, and vice versa
- In spring and autumn, a sharp drop in the barometer foreshadows windy weather. In summer, in extreme heat, it predicts a thunderstorm. In winter, especially after prolonged frosts, a rapid drop in the mercury column indicates an upcoming change in wind direction, accompanied by thaw and rain. On the contrary, an increase in mercury during prolonged frosts foretells snowfall.
- Frequent fluctuations in the level of the mercury column, sometimes rising, sometimes falling, should in no case be considered as a sign of the approach of a long period; period of dry or rainy weather. Only a gradual and slow fall or rise in the mercury heralds the onset of a long period of stable weather.
- When, at the end of autumn, after a long period of wind and rain, the barometer begins to rise, this heralds a north wind at the onset of frost.
Here are the general conclusions that can be drawn from the readings of this valuable device. Dick Sand was an excellent judge of the barometer's predictions and was convinced many times how correct they were. Every day he consulted his barometer so as not to be taken by surprise by changes in the weather.”
I made observations of weather changes and atmospheric pressure. And I became convinced that this dependence exists.
| date | Temperature, °C | Precipitation, | Atmospheric pressure, mm Hg. | Cloudiness |
| 28.01.2017 | -3 | 765 | It's clear | |
| 29.01.2017 | -6 | 761 | Mainly cloudy | |
| 30.01.2017 | -4 | 767 | It's clear | |
| 31.01.2017 | -5 | 763,5 | Mainly cloudy | |
| 01.02.2017 | -6 | 751 | Mainly cloudy | |
| 02.02.2017 | -12 | 758 | Mainly cloudy | |
| 03.02.2017 | -12 | 753 | Mainly cloudy | |
| 04.02.2017 | -5 | 754 | It's clear | |
| 05.02.2017 | -16 | 755 | It's clear | |
| 06.02.2017 | -23 | 764 | It's clear | |
| 07.02.2017 | -21 | 769 | It's clear | |
| 08.02.2017 | -15 | 765 | Mainly cloudy | |
| 09.02.2017 | 0 | 768 | It's clear | |
| 10.02.2017 | 0 | 764 | Mainly cloudy |
Isobaths
Isobats are used in cartography, denoting lines that connect marks that characterize equal atmospheric pressure. The definition may seem complicated, but the examples below will make everything clear. There are 2 types of such points: closed and open.
An example of a closed isobar is a cyclone. Inside such an isobath, there is low atmospheric pressure, since a large amount of precipitation falls, which forms evaporation, and also interferes with the rapid heating of the surface. As a result, an area of low pressure is formed inside, and it is surrounded by an area of high pressure.
An example of an open isobar is an anticyclone. In this case, in the center there is an area with high atmospheric pressure, and outside it there is an area with low atmospheric pressure. These situations arise when there is no precipitation inside the isobath for a long time, and the surface warms up easily and quickly. This is one of the sources of high atmospheric pressure. Such isobaths are most often found in the South Pacific or North Atlantic Ocean.
Instruments for measuring atmospheric pressure
For scientific and everyday purposes, you need to be able to measure atmospheric pressure. There are special devices for this - barometers . Normal atmospheric pressure is the pressure at sea level at a temperature of 15 °C. It is equal to 760 mm Hg. Art. We know that when the altitude changes by 12 meters, the atmospheric pressure changes by 1 mmHg. Art. Moreover, with increasing altitude, atmospheric pressure decreases, and with decreasing altitude, it increases.
The modern barometer is made liquidless. It's called an aneroid barometer. Metal barometers are less accurate, but not as bulky or fragile.
An aneroid barometer is a very sensitive instrument. For example, when climbing to the top floor of a nine-story building, due to differences in atmospheric pressure at different altitudes, we will find a decrease in atmospheric pressure by 2-3 mmHg. Art.
A barometer can be used to determine the flight altitude of an aircraft. Such a barometer is called a barometric altimeter or altimeter . The idea of Pascal's experiment formed the basis for the design of the altimeter. It determines the height above sea level by changes in atmospheric pressure.
When observing the weather in meteorology, if it is necessary to record fluctuations in atmospheric pressure over a certain period of time, they use a recording device - a barograph .
Storm Glass (storm glass, Dutch storm - “storm” and glass - “glass”) is a chemical or crystal barometer consisting of a glass flask or ampoule filled with an alcohol solution in which camphor, ammonia and ammonia are dissolved in certain proportions. potassium nitrate.
This chemical barometer was actively used during his sea voyages by the English hydrographer and meteorologist, Vice Admiral Robert Fitzroy, who carefully described the behavior of the barometer; this description is still used today. Therefore, stormglass is also called the “Fitzroy Barometer”. From 1831–36, Fitzroy led the oceanographic expedition on HMS Beagle, which included Charles Darwin.
The barometer works as follows. The flask is hermetically sealed, but, nevertheless, the birth and disappearance of crystals constantly occurs in it. Depending on upcoming weather changes, crystals of various shapes form in the liquid. Stormglass is so sensitive that it can predict sudden weather changes 10 minutes in advance. The principle of operation has never received a complete scientific explanation. The barometer works better when located near a window, especially in reinforced concrete houses; probably in this case the barometer is not so shielded.
A baroscope is a device for monitoring changes in atmospheric pressure. You can make a baroscope with your own hands. To make a baroscope, the following equipment is required: A glass jar with a volume of 0.5 liters.
- A piece of film from a balloon.
- Rubber ring.
- Lightweight straw arrow.
- Wire for fastening the arrow.
- Vertical scale.
- Device body.
Dependence of liquid pressure on the height of the liquid column in liquid barometers
When atmospheric pressure changes in liquid barometers, the height of the liquid column (water or mercury) changes: when the pressure decreases, it decreases, when the pressure increases, it increases. This means that there is a dependence of the height of the liquid column on atmospheric pressure. But the liquid itself presses on the bottom and walls of the vessel.
The French scientist B. Pascal in the middle of the 17th century empirically established a law called Pascal's law:
Pressure in a liquid or gas is transmitted equally in all directions and does not depend on the orientation of the area on which it acts.
To illustrate Pascal's law, the figure shows a small rectangular prism immersed in a liquid. If we assume that the density of the prism material is equal to the density of the liquid, then the prism must be in a state of indifferent equilibrium in the liquid. This means that the pressure forces acting on the edge of the prism must be balanced. This will happen only if the pressures, that is, the forces acting per unit surface area of each face, are the same: p1 = p2 = p3 = p.
The pressure of the liquid on the bottom or side walls of the vessel depends on the height of the liquid column. The pressure force on the bottom of a cylindrical vessel of height h and base area S is equal to the weight of the liquid column mg, where m = ρghS is the mass of the liquid in the vessel, ρ is the density of the liquid. Therefore p = ρghS / S
In accordance with Pascal’s law, the liquid exerts the same pressure at depth h on the side walls of the vessel. The pressure of the liquid column ρgh is called hydrostatic pressure .
Many devices that we encounter in life use the laws of liquid and gas pressure: communicating vessels, water supply, hydraulic press, sluices, fountains, artesian well, etc.
Dependence on air temperature
Air temperature is one of the key factors in the formation of atmospheric pressure. When heated, air expands, becomes denser and at the same time lighter. As a result, atmospheric pressure decreases. If we talk about cold air, then it becomes, on the contrary, heavier, increasing atmospheric pressure. Therefore, when we talk about air temperature, we need to understand that a change in this indicator simultaneously entails a change in air pressure.
To understand how this movement (primarily the replacement of air) occurs through temperatures, we can consider circulation into the premises. The air, heated by the battery, rises up to the ceiling. It stays there for some time, cools down and goes down. Then it gets back into the battery, heats up and rises again. This happens in a closed cycle.