Chronic myeloid leukemia is a malignant blood disease in which there is excessive formation and impaired maturation of granulocytes in the bone marrow. A unique feature of this type of leukemia is the presence in tumor cells of a specific marker - the Philadelphia chromosome. It is formed as a result of the t(9;22) translocation, in which one fragment of chromosome 22 changes places with a fragment of chromosome nine. As a result, a chimeric gene is formed that disrupts the process of division and maturation of myeloid cells.
- Causes of development of chronic myeloid leukemia
- Pathogenesis of chronic myeloid leukemia
- Stages of chronic myeloid leukemia
- Symptoms and signs of chronic myeloid leukemia
- Diagnosis of chronic myeloid leukemia
- Treatment of chronic myeloid leukemia
- Prognosis and prevention of chronic myeloid leukemia
Pathogenesis of chronic myeloid leukemia
The Philadelphia chromosome is the result of a reciprocal translocation between chromosomes 9 and 22. In this case, the ABL oncogene from chromosome 9 is transferred to chromosome 22 and attached to the BCR gene. As a result, a hybrid BCR-ABL gene is formed, which regulates the synthesis of a special oncogenic protein - tyrosine kinase bcr-abl. This oncoprotein disrupts the process of cell division, protects tumor cells from programmed death (apoptosis) and disrupts their adhesion to the bone marrow stroma, due to which immature cells enter the bloodstream.
Most often, chronic myeloid leukemia develops as a result of a mutation in a pluripotent hematopoietic stem cell, which is located in the bone marrow, but there are cases when the primary focus is in the liver or spleen.
In chronic myeloid leukemia, the pathology of the granulocytic lineage mainly predominates, but all lines of hematopoiesis can suffer - erythrocyte lineage, monocytes, etc. Healthy stem cells are preserved and can give rise to new hematopoiesis after chemotherapy.
General characteristics
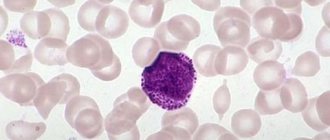
Myelocytes are mature cells, the size of which varies from 12 to 30 microns.
They have a core that can be:
- Clinical blood analysis: from light microscope to hematological analyzers
- oval;
- kidney-shaped;
- round.
Neutrophils act as the nucleus, and in the vast majority of cases it is located eccentrically. In addition, it has the ability to acquire a red-violet hue, which is determined by its maturation.
If any disease develops in the body, mature leukocytes come out to fight it. However, the more complex the pathology or the longer it lasts, the more young cells the immune system is forced to use. When the supply of leukocytes is completely depleted, myelocytes begin to fight the disease.
This is what determines their appearance in the blood, which in the normal state of affairs should not happen, since they are formed and subsequently localized only in the bone marrow.
In total, there are 3 types of such components, which is why clinicians distinguish:
- eosinophilic myelocyte - has weakly basophilic protoplasm, consisting of a large number of pinkish-red large grains;
- basophilic - characterized by the presence of oxyphilic protoplasm, colored violet;
- neutrophil myelocyte - considered a more mature cell than the previous type - includes pink protoplasm.
The appearance of such substances in the blood always indicates the progression of the disease in the body. This means that the norm is the complete absence of myelocytes in the blood.
However, in human bone marrow there may be myelocytes:
- neutrophilic – from 5 to 10%;
- basophilic – from 0.2 to 1%;
- eosinophilic – from 0.5 to 2%.
Clinicians do not consider this concentration to be pathological.
Myelocytes
Stages of chronic myeloid leukemia
During chronic myeloid leukemia, 4 stages are distinguished, which reflect the progression of the pathology. In this case, the disease can be detected on any of them.
Chronic or preclinical stage - has no clinical symptoms; the diagnosis can be suspected by a general blood test, which patients can take either as part of a medical examination or for the diagnosis of another disease. In most cases, chronic myeloid leukemia is detected at this stage.
Acceleration phase, or progression stage. During this period, the number of tumor granulocytic cells increases, and various symptoms appear, for example, weakness or bone pain. Objectively, there is an increase in the number of blasts in the blood or bone marrow up to 15-29%, the number of basophils increases (more than 20%), thrombocytopenia or thrombocytosis is detected (more than 1000×10 9 ).
Blast crisis is a phase during which there is a sharp increase in the number of blast cells (more than 30%) and the disease in its course resembles aggressive acute leukemia.
The patient is in serious condition. There is an increase in temperature, persistent infections, bleeding, and leukemic skin lesions. At this stage, leukemia is difficult to treat.
The phase of chronic myeloid leukemia is necessarily assessed when making a diagnosis and then rechecked as the pathology progresses and the need to change treatment.
Publications in the media
Acute leukemia is a malignant disease of the hematopoietic system; morphological substrate - blast cells. Frequency. 13.2 cases per 100,000 population among men and 7.7 cases per 100,000 population among women.
CLASSIFICATION FAB classification (Franco-American-British) is based on the morphology of leukemic cells (structure of the nucleus, ratio of the sizes of the nucleus and cytoplasm) • Acute myeloblastic (non-lymphoblastic) leukemia (AML) •• M0 - without cell maturation, myelogenous differentiation is proven only immunologically •• M1 - without cell maturation •• M2 - AML with cell differentiation, •• M3 - promyelocytic •• M4 - myelomonocytic •• M5 - monoblastic leukemia •• M6 - erythroleukemia •• M7 - megakaryoblastic leukemia • Acute lymphoblastic leukemia (ALL): •• L1 - without cell differentiation (morphologically homogeneous cells) •• L2 - with cell differentiation (morphologically heterogeneous population of cells) •• L3 - Burkett-like leukemias • Undifferentiated leukemia - this category includes leukemias whose cells cannot be identified as myeloblastic or lymphoblastic (neither chemical, neither by immunological methods) • Myelopoietic dysplasia •• Refractory anemia without blastosis (in the bone marrow blasts and promyelocytes <10%) •• Refractory anemia with blastosis (in the bone marrow blasts and promyelocytes 10 30%) •• Refractory anemia with an excess of blasts in transformation •• Chronic myelomonocytic leukemia.
REAL classification (Revised European American classification of Lymphoid neoplasms), revised (European American) classification of lymphoid hemoblastoses • Pre B cell tumors •• Pre B lymphoblastic leukemia/lymphoma • Pre T cell tumors •• Pre T lymphoblastic leukemia/lymphoma • Peripheral B tumors cells •• chronic lymphocytic leukemia/small lymphocyte lymphoma •• Lymphoplasmocytic lymphoma •• Mantle cell lymphoma •• Follicular lymphoma •• Marginal zone cell lymphoma •• Hairy cell leukemia •• Plasmacytoma/plasmocytic myeloma •• Diffuse lymphoma of large lymphocytes• • Burkett's lymphoma • Tumors of peripheral T cells and NK cells •• T cell chronic lymphocytic leukemia •• Leukemia of large granular lymphocytes •• Mycosis fungoides and Sezary syndrome T cell lymphoma •• Angioimmunoblastic T cell lymphoma •• Angiocentric lymphoma (lymphoma of NK and T cells) •• Intestinal T cell lymphoma •• Leukemia/adult T cell lymphoma •• Anaplastic large cell lymphoma
Variants of AML (WHO classification, 1999) • AML with t(8;21)(q22;q22) • AML with t(15;17) (q22;q11 12) • Acute myelomonoblastic leukemia • AML with pathological bone marrow eosinophilia (inv( 16)(p13q22) or t(16;16) (p13;q11) • AML with 11q23 (MLL) defects • Acute erythroid leukemia • Acute megakaryocytic leukemia • Acute basophilic leukemia • Acute panmyelosis with myelofibrosis • Acute biphenotypic leukemia • AML with multilineage dysplasia • Secondary AML.
Immunohistochemical study (determination of cellular phenotype) is necessary to clarify the immunological variant of leukemia, which affects the treatment regimen and clinical prognosis
• Acute lymphoblastic leukemia (247640, , mutation of somatic cells) - 85% of all cases, accounts for up to 90% of all childhood leukemias. It develops quite rarely in adults. Cytochemical reactions: positive for terminal deoxynucleotidyl transferase; negative for myeloperoxidosis, glycogen. The use of cell membrane markers made it possible to identify subtypes •• B-cell - 75% of all cases •• With the absence of rosette formation •• T-cell •• Other variants (rare). Differential diagnosis of subtypes is important for prognosis, because T-cell variants are difficult to treat.
• Acute myeloid leukemia occurs more often in adults, the subtype depends on the level of cell differentiation. In most cases, the myeloblast clone originates from hematopoietic stem cells capable of multiple differentiation into colony-forming units of granulocytes, erythrocytes, macrophages or megakaryocytes, therefore, in most patients, malignant clones do not show signs of lymphoid or erythroid lineages •• AML is most often observed; has four variants (M0 - M3) •• M0 and M1 - acute leukemia without cell differentiation •• M2 - acute with cell differentiation •• M3 - promyelocytic leukemia, characterized by the presence of abnormal promyelocytes with giant granules; often combined with DIC caused by the thromboplastic effect of granules, which casts doubt on the advisability of using heparin in therapy. The prognosis for M3 is less favorable than for M0–M1 •• Myelomonoblastic and monoblastic leukemia (M4 and M5, respectively) are characterized by a predominance of non-erythroid cells such as monoblasts. M4 and M5 account for 5–10% of all AML cases. A common symptom is the formation of extramarrow foci of hematopoiesis in the liver, spleen, gums and skin, hyperleukocytosis exceeding 50–100109/l. Sensitivity to therapy and survival are lower than with other types of acute myeloblastic leukemia •• Erythroleukemia (M6). A variant of acute myeloblastic leukemia, accompanied by increased proliferation of erythroid precursors; characterized by the presence of abnormal blast nucleated red blood cells. The effectiveness of treatment for erythroleukemia is similar to the results of treatment for other subtypes or slightly lower • Megakaryoblastic leukemia (M7) is a rare variant combined with bone marrow fibrosis (acute myelosclerosis). Doesn't respond well to therapy. The prognosis is unfavorable. Pathogenesis is caused by the proliferation of tumor cells in the bone marrow and their metastasis to various organs. Inhibition of normal hematopoiesis is associated with two main factors: • damage and displacement of the normal hematopoietic germ by poorly differentiated leukemic cells • production of inhibitors by blast cells that suppress the growth of normal hematopoietic cells.
Stages of acute leukemia • Primary active phase • Remission (with treatment) - complete clinical and hematological •• The content of blasts in the bone marrow is less than 5% with normal cellularity •• There is no proliferative syndrome in the clinical picture • Relapse (early and late) •• Isolated bone marrow - the content of blasts in the bone marrow is more than 25% •• Extramarrow ••• Neuroleukemia (neurological symptoms, cytosis of more than 10 cells, blasts in the cerebrospinal fluid) ••• Testicular (increase in the size of one or two testicles, the presence of blasts is confirmed by cytological and histological studies) •• Mixed • Terminal phase (in the absence of treatment and resistance to therapy)
The clinical picture of acute leukemia is determined by the degree of infiltration of the bone marrow by blast cells and inhibition of hematopoietic sprouts • Inhibition of bone marrow hematopoiesis •• Anemic syndrome (myelophthisis anemia) •• Hemorrhagic syndrome (due to thrombocytopenia, skin hemorrhages are noted - petechiae, ecchymoses; bleeding from the mucous membranes - nosebleeds, internal bleeding) •• Infections (leukocyte dysfunction) • Lymphoproliferative syndrome •• Hepatosplenomegaly •• Enlarged lymph nodes • Hyperplastic syndrome •• Bone pain •• Lesions of the skin (leukemids), meninges (neuroleukemia) and internal organs • Intoxication syndrome • • Weight loss •• Fever •• Hyperhidrosis •• Severe weakness.
The diagnosis of acute leukemia is confirmed by the presence of blasts in the bone marrow. To identify the subtype of leukemia, histochemical, immunological and cytogenetic research methods are used.
Laboratory tests • In peripheral blood, the level of leukocytes can vary from severe leukopenia (below 2.0109/l) to hyperleukocytosis; anemia, thrombocytopenia; the presence of blast cells up to total blastosis • Hyperuricemia due to the accelerated life cycle of cells • Hypofibrinogenemia and increased content of fibrin destruction products due to concomitant DIC. The influence of drugs. GCs should not be prescribed until a definitive diagnosis has been made. High sensitivity of blast cells to prednisolone leads to their destruction and transformation, making diagnosis difficult. Treatment is complex; the goal is to achieve complete remission. Currently, hematology centers use various chemotherapy protocols based on the principles of polychemotherapy and intensification of treatment.
• Chemotherapy consists of several stages •• Induction of remission ••• For ALL - one of the schemes: a combination of vincristine intravenously weekly, prednisolone orally daily, daunorubicin and asparaginase for 1-2 months continuously ••• For AML - a combination of cytarabine in /drip or subcutaneous injection, daunorubicin intravenously, sometimes in combination with thioguanine. More intensive post-induction chemotherapy, which destroys remaining leukemia cells, increases the duration of remission •• Consolidation of remission: continuation of systemic chemotherapy and prevention of neuroleukemia in ALL (endolumbar methotrexate in ALL combined with radiation therapy to the brain with involvement of the spinal cord [C1-C2]) •• Maintenance therapy: periodic courses of remission reinduction.
• AML M3 is treated with retinoic acid (tretinoin). • Bone marrow transplantation is the method of choice for acute myeloblastic leukemia and for relapses of all acute leukemia. The main condition for transplantation is complete clinical and hematological remission (the content of blasts in the bone marrow is less than 5%, the absence of absolute lymphocytosis). Before surgery, chemotherapy can be administered in ultra-high doses, alone or in combination with radiation therapy (to completely destroy leukemia cells) •• The optimal donor is an identical twin or sibling; Donors with a 35% Ag HLA match are more often used. In the absence of compatible donors, autotransplantation of bone marrow taken during remission is used •• The main complication is graft-versus-host disease. It develops as a result of transplantation of donor T-lymphocytes, which recognize the recipient's Ags as foreign and cause an immune reaction against them. An acute reaction develops within 20–100 days after transplantation, a delayed reaction occurs after 6–12 months ••• The main target organs are the skin (dermatitis), gastrointestinal tract (diarrhea) and liver (toxic hepatitis) ••• Treatment is long-term, usually limited prescribing combinations of prednisolone, cyclosporine and small doses of azathioprine •• The course of the post-transplantation period is also influenced by preparatory treatment regimens, the development of interstitial pneumonia, and graft rejection (rarely).
• Replacement therapy •• Transfusion of red blood cells to maintain the Hb level not lower than 100 g/l. Transfusion conditions: unrelated donor, use of leukocyte filters •• Transfusion of fresh platelets (reduces the risk of bleeding). Indications: platelet count less than 20109/l; hemorrhagic syndrome with platelet content less than 50109/l.
• Prevention of infections is the main condition for the survival of patients with neutropenia resulting from chemotherapy •• Complete isolation of the patient •• Strict sanitary and disinfection regime - frequent wet cleaning (up to 4-5 times a day), ventilation and quartzing of rooms; use of disposable instruments, sterile clothing for medical personnel •• Prophylactic use of antibiotics, antifungal and antiviral drugs (if the content of segmented neutrophils is less than 0.5109/l, prevention of Pneumocystis pneumonia is indicated) ••• If the body temperature increases, clinical and bacteriological studies are carried out and immediately begin treatment with combinations of broad-spectrum bactericidal antibiotics: cephalosporins, aminoglycosides and semi-synthetic penicillins ••• For secondary increases in body temperature that occur after treatment with broad-spectrum antibiotics, antifungal agents (amphotericin B) are empirically used •• Colony-stimulating agents can be prescribed for the prevention and treatment of neutropenia factors (for example, molgramity).
Prognosis • The prognosis for children with acute lymphocytic leukemia is good: 95% or more experience complete remission. 70–80% of patients have no manifestations of the disease for 5 years and are considered cured. If a relapse occurs, in most cases a second complete remission can be achieved. Patients in second remission are candidates for bone marrow transplantation with a long-term survival rate of 35–65% • The prognosis for patients with acute myeloid leukemia is poor. 75% of patients receiving adequate treatment using modern chemotherapy regimens achieve complete remission, 25% of patients die (remission duration is 12–18 months). There are reports of cure in 20% of cases with continued intensive therapy after remission. The prognosis for the M3 variant of AML improves with treatment with retinoic acid drugs. Patients under 30 years of age can undergo bone marrow transplantation after achieving the first complete remission. 50% of young patients who have undergone allogeneic transplantation develop long-term remission. Encouraging results have also been obtained with autologous bone marrow transplants.
Age characteristics • Children •• 80% of all acute leukemias are ALL •• Adverse prognostic factors for ALL ••• Age of the child under 1 year and over 10 years ••• Male gender ••• T-cell variant of ALL ••• Leukocyte content at the time of diagnosis more than 20109/l ••• Lack of clinical and hematological remission against the background of induction •• Prognosis and course. 80% of clinical and hematological remission. 5-year survival rate is 40–50%.
• Elderly . Reduced tolerance to allogeneic bone marrow. The maximum age for transplantation is 50 years. Autologous transplantation can be performed in patients over 50 years of age in the absence of organ damage and general somatic well-being.
Abbreviations • MDS - myelodysplastic syndrome • ALL - acute lymphoblastic leukemia • AML - acute myeloid leukemia.
ICD-10 • C91.0 Acute lymphoblastic leukemia • C92 Myeloid leukemia [myeloid leukemia] •• C93.0 Acute monocytic leukemia
Symptoms and signs of chronic myeloid leukemia
The symptoms of chronic myeloid leukemia are characterized by a variety of clinical manifestations and depend on the aggressiveness of the course and stage of the disease. In general, there may be several syndromes:
- Tumor intoxication syndrome. It manifests itself as weakness, weight loss, and loss of appetite that is inadequate to the current state. There may be fever, increased sweating, itchy skin, and bone pain.
- Tumor proliferation syndrome. It develops with an active increase in the number of malignant cells infiltrating the liver and spleen. In this case, patients note pain and heaviness in the left side.
- Anemic syndrome - develops with a decrease in the number of red blood cells and hemoglobin levels. Manifested by weakness, shortness of breath, increased fatigue during routine physical activity. A decrease in pressure, pallor of the skin and mucous membranes, dizziness, and tachycardia may be observed.
- Disorders of the blood clotting system - thrombosis and hemorrhage (bleeding). The cause of thrombosis is most often thrombocytosis (increased platelet level above 1000 × 10 9 / l). In this case, thrombophlebitis, heart attacks, and strokes may occur. Hemorrhagic manifestations are characterized by an increase in bleeding time after injury, as well as the formation of a petechial hemorrhagic rash. They develop against the background of a critical decrease in platelet levels.
Diagnosis of chronic myeloid leukemia
In most cases, chronic myeloid leukemia is an incidental finding that is discovered during examination for another reason. It can be suspected by a general blood test, in particular by an increase in the number of leukocytes and the predominance of granulocytic hematopoiesis in the formula. In this case, not only the number of neutrophils, but also basophils and eosinophils may increase. There may be mild anemia or abnormal platelet counts.
If the doctor suspects chronic myeloid leukemia, the patient is referred for further examination - puncture and bone marrow biopsy. To confirm the diagnosis, it is necessary to conduct a standard cytogenetic study of the bone marrow for the presence of the Philadelphia chromosome. At least 20 metaphases are studied. If cytogenetics is not possible, fluorescent in situ hybridization is used to identify the chimeric gene. The expression of the chimeric gene in peripheral blood cells is also determined by PCR. If a typical transcript is not detected, and there are clinical and hematological signs of chronic myeloid leukemia, detection of rarer mutations is indicated - BCR-ABLp190, p230.
In the blast crisis phase, immunophenotyping of blast cells and cytological and biochemical examination of the cerebrospinal fluid are performed. When chronic myeloid leukemia is detected in the activation phase or blast crisis, the search for the Philadelphia chromosome can be carried out by sequencing the genetic material of blood cells.
Treatment of chronic myeloid leukemia
At the initial stage, before obtaining cytogenetic confirmation of the diagnosis (as we already know, chronic myeloid leukemia is diagnosed in the presence of the Philadelphia chromosome), symptomatic therapy with hydroxyurea is prescribed. Its goal is to reduce the overall level of white blood cells and platelets. If there is intolerance to the drug or insufficient reduction in platelet levels, anagrelide can be used. If there are signs of leukostasis (encephalopathy, visual disturbances, renal dysfunction), leukapheresis is performed.
After cytogenetic confirmation of the diagnosis, specific antitumor therapy is prescribed. The main drugs are tyrosine kinase inhibitors (TKIs). The dosage is selected depending on the level of leukocytes. At the initial stage, to prevent tumor lysis syndrome, enhanced hydration is necessary (additional fluid administration in a volume of 2-2.5 l/m2, if there are no contraindications from the cardiovascular system) and the administration of allopurinol.
The goal of specific treatment for chronic myeloid leukemia is to suppress the tumor cell clone, reduce the risk of pathology progression and prolong the patient’s life to values comparable to general population indicators. With the introduction of TKIs into practice, these tasks became quite feasible and not only made it possible to increase the overall survival of such patients several times, but also to eliminate lifelong medication use and transfer to dynamic observation in patients with a good molecular tumor response.
Currently, according to accepted treatment protocols, TKIs should be prescribed to all patients with newly diagnosed chronic myeloid leukemia. Their mechanism of action is based on the blockade of the ATP-binding pocket of the pathological molecule BCR-ABL, which deprives this protein of tyrosine kinase activity, which stimulates excessive division of tumor cells. With constant use of TKIs, the tumor clone undergoes reduction, which makes it possible to restore normal hematopoiesis.
In Russia, the following drugs from the TKI group are registered for first-line therapy:
- imatinib,
- nilotinib,
- dasatinib.
Imatinib
Imatinib has selective activity against BCR-ABL tyrosine kinase and several other tyrosine kinases. It is prescribed in long courses and must be taken daily. The initial dosage is 400 mg per day for the chronic phase of myeloid leukemia, and 600 mg/day for the accelerated phase or blast crisis. The dosage does not depend on the patient’s height, gender and body weight. The drug is available in tablet form or capsules. Can be used on an outpatient basis. If the result of therapy is unsatisfactory, the dosage can be increased, and if toxic complications develop, it can be reduced.
Nilotinib
Nilotinib is a highly selective BCR-ABL tyrosine kinase inhibitor. It was developed based on the imatinib molecule and modified to increase affinity for BCR-ABL tyrosine kinase. Available in capsules. The dosage for chronic phase therapy is 600 mg/day, for acceleration phase therapy - 800 mg/day. The drug is taken 2 times a day with an interval of 12 hours strictly on an empty stomach, since food increases the bioavailability of the drug, which increases its concentration in plasma and can provoke the development of toxic complications. If the therapeutic effect in the treatment of the chronic phase is insufficient, the dosage may be increased to 800 mg/day. If complications develop, the dose is reduced.
Dasatinib
Dasatinib has activity against many tyrosine kinases, including mutant BCR-ABL. Penetrates the blood-brain barrier. Available in forms for oral use. The recommended dosage is 100 mg/day for the chronic phase and 140 mg/day for the accelerated phase and blast crisis. If chronic phase therapy is insufficiently effective, the dosage may be increased to 140 mg/day. If toxic complications develop, it is reduced to 80 mg/day.
Bosutinib
Bosutinib is a relatively new drug, registered in Russia in 2014 and is used for second and subsequent line therapy in cases of intolerance or ineffectiveness of the above drugs. The standard daily dose is 500 mg, which can be increased to 600 mg if necessary.
The choice of first-line drug is carried out individually for each patient. This takes into account the phase of leukemia, the sensitivity of the tumor clone with individual mutations, the toxicity profile of each drug and the presence of concomitant diseases in the patient.
Determination of the spectrum of mutations is carried out when the pathology manifests itself in the acceleration phase or blast crisis, or when therapy with the selected drug is ineffective and there is a need to change the drug, since there is a possibility of the emergence of resistant clones. For example, mutations F317L/V, T315A, V299L cause low sensitivity to dasatinib, so it is changed to nilotinib. Mutations Y253H, E255K/V, F359V/C, on the contrary, make tumor cells resistant to nilotinib, so dasatinib is indicated for such patients.
Mutations E255K/V, G250E, V299L cause resistance to bosutinib. The presence of the T3151 mutation determines resistance to all types of TKIs, so allogeneic hematopoietic stem cell transplantation is recommended for such patients. Ponatinib therapy is also a possible option, but it has not yet been registered in Russia.
The effect of first-line therapy can be classified into one of three groups:
- Optimal answer. This is a good result, which allows us to hope for a long period of disease-free survival (7-8 years or more). The criteria for achieving optimal response are complete hematologic response within 3 months, complete cytogenetic response within 6 months, and major molecular response within 12-18 months.
- Warning. In the presence of warning factors, careful monitoring of the patient's condition and readiness to change the treatment regimen are required. Prevention factors include a high-risk group for chronic myeloid leukemia, a more than 10-fold increase in the transcription level of the mutant gene, and the presence of additional chromosomal abnormalities in cells with the Philadelphia chromosome.
- Therapy failure. This includes the progression of the disease, the emergence of new mutations, and the appearance of additional chromosomal abnormalities in cells with the Philadelphia chromosome. A change in therapy is required.
Other treatments for chronic myeloid leukemia
Donor stem cell transplantation is indicated for patients who have failed second-line therapy and for patients with the T315I mutation. Patients intolerant of TKIs and unable to undergo transplantation can be treated with hydroxyurea, interferons and cytostatics.
Page 1 of 5
Acute leukemia is the most dangerous and rapid blood disease. In acute leukemia, breakdown occurs at the level of the youngest blood cells, precursor cells, blast cells, which are one of the subtypes of white blood cells, leukocytes. The word blast comes from the word blastos, which in Greek means “sprout, germ, shoot.” Blasts are fast growing cells. A healthy blast cell eventually develops into some useful blood cell. Normally, the number of blasts does not exceed 1% of all bone marrow cells. They almost never go into the bloodstream. If a person urgently needs a large number of some blood cells (for example, leukocytes during a severe infection), then the body can produce more blasts, but still their relative number rarely exceeds 10%.
The blasts that multiply in acute leukemia are different. They can be compared to hooligans-parasites in human society, because they do not work, feed on the body’s reserves, and instead of developing themselves into useful cells, they quickly reproduce their own kind. Leukemia blasts displace healthy blood cells from their home inside the bones and take up residence there themselves. Because of this, the renewal process of normal blood is disrupted, and its deficiency begins: hemoglobin, platelets, and healthy leukocytes quickly decrease. The patient begins to weaken, spontaneous bleeding and high fever may appear. In a blood test, the doctor sees a decrease in hemoglobin and platelets and a sharp increase in leukocytes due to tumor blast cells. Most tumor cells are in the bone marrow.
Basically, doctors distinguish two types of blasts: lymphoblasts and myeloblasts. Depending on the type of blasts, leukemias are classified as lymphoblastic and myeloblastic (non-lymphoblastic). It depends on which of the two paths of blood cell development the accident occurred at the beginning.
Normally, highly specialized cells of the immune system, B- and T-lymphocytes, are formed from healthy lymphoblasts (precursor cells). The tasks of B and T cells can be compared to the tasks of special forces, which operate with precision and efficiency. From myeloblasts, other types of cells are subsequently obtained: red blood cells, platelets, granulocytes. Red blood cells (erythrocytes) - carry oxygen, platelets are responsible for blood clotting if, for example, we are injured. Granulocytes are the “combined arms” part of the immune system; they act in more massive attacks.
If a breakdown occurs, then normal cells do not mature, and blasts remain underdeveloped. By the appearance of the blast, as well as with the help of flow cytometry, it is possible to determine what kind of cell could arise from it. This is important for choosing treatment and understanding how successful the treatment will be. Doctors designate the type of progenitor cells in the diagnosis, for example: acute B-lymphoblastic leukemia (leukemia from B-lymphocyte precursor cells), acute monoblastic leukemia ( leukemia from monocyte precursor cells), etc.
The diagnosis of “ acute leukemia ” is made if it is found that there are more than 20% of blasts in the patient’s bone marrow. To understand what kind of leukemia a patient has, flow cytometry of blasts is done.
Acute leukemias are very different. Leukemia , for example, can appear “out of the blue,” that is, in a person who has never had any illness worse than a runny nose. Such leukemias usually affect children and young people. In older people, acute leukemia is usually secondary. This means that as a result of some reasons (chemotherapy for other diseases, radiation or poisoning with certain chemicals), hematopoiesis may be disrupted, that is, the so-called myelodysplastic syndrome appears (we have already described it in other sections of our site). The last stage of myelodysplastic syndrome is secondary acute leukemia . Primary leukemias are more aggressive than secondary ones, but all leukemias are treated according to the same principles. To completely cure a patient, it is often necessary to undergo a transplant of donor blood stem cells.
about some types of acute leukemia in this chapter:
- acute myeloblastic leukemia;
- acute lymphoblastic leukemia;
- myeloid sarcoma;
- acute leukemia of mixed phenotype.
- Back
- Forward >>
Prognosis and prevention of chronic myeloid leukemia
There are no specific methods of prevention, since the reasons for the formation of mutations that cause chronic myeloid leukemia are unknown. As for the prognosis, it all depends on the patient’s age, response to treatment and the possibility of allogeneic transplantation. In general, the situation is quite favorable and allows us to hope for a life expectancy comparable to general population indicators. In some patients, it is possible to achieve stable remission and avoid lifelong use of TKIs with regular follow-up.
At the Euroonco clinic, the treatment of myeloid leukemia complies with all modern treatment protocols. In difficult cases, the decision is made by a council of specialists, and when remission is achieved, we pay special attention to regular monitoring of the patient, which also allows us to improve treatment results.
Book a consultation 24 hours a day
+7+7+78