4.1. Two-stage test in test tubes with antiglobulin
4.2. Compatibility test on a plane at room temperature
4.3. Indirect Coombs test
4.4. Compatibility test using 10% gelatin
4.5. Compatibility test using 33% polyglucin
An individual compatibility test allows you to make sure that the recipient does not have antibodies directed against the donor’s red blood cells and thus prevents the transfusion of red blood cells that are incompatible with the patient’s blood.
The compatibility test, performed on a plane at room temperature, is aimed at identifying complete group agglutinins of the AB0 system, MNSs, Lewis, etc. in the recipient. The compatibility test using 10% gelatin, 33% polyglucin, an indirect Coombs test is intended to identify incomplete group antibodies. A two-stage test in test tubes with antiglobulin involves the detection of both antibodies, including group hemolysins.
The most sensitive and recommended is a two-stage test in tubes with antiglobulin, then a combination of two tests - a flat test at room temperature and an indirect Coombs test. Instead of the indirect Coombs test, a conglutination reaction with 10% gelatin or a conglutination reaction with 33% polyglucin can be used. The last test is inferior in sensitivity to the first two, but takes less time.
4.1. Two-stage test in test tubes with antiglobulin
First stage.
Add 2 volumes (200 µl) of the recipient's serum and 1 volume (100 µl) of a 2% suspension of thrice-washed donor erythrocytes suspended in saline or LISS (low ionic strength solution) into a labeled tube. The contents of the tube are mixed and centrifuged at 2500 rpm (about 600 g) for 30 s. The presence of hemolysis in the supernatant is then assessed, after which the erythrocyte pellet is resuspended by lightly tapping the bottom of the tube with a fingertip, and the presence of erythrocyte agglutination is determined. In the absence of pronounced hemolysis and/or agglutination, proceed to the second stage of the test using antiglobulin serum.
Second phase.
The tube is placed in a thermostat at 37°C for 30 minutes, after which the presence of hemolysis and/or red blood cell agglutination is again assessed. Then the red blood cells are washed three times with saline, 2 volumes (200 μl) of antiglobulin serum for the Coombs test are added and mixed. The tubes are centrifuged for 30 s, the red blood cell sediment is resuspended and the presence of agglutination is assessed.
The results are recorded with the naked eye or through a magnifying glass. Severe hemolysis and/or agglutination of erythrocytes indicates the presence in the recipient's serum of group hemolysins and/or agglutinins directed against the donor's erythrocytes and indicates incompatibility of the blood of the recipient and the donor. The absence of hemolysis and/or agglutination of red blood cells indicates the compatibility of the blood of the recipient and the donor.
4.2. Compatibility test on a plane at room temperature
Apply 2-3 drops of the recipient's serum to the plate and add a small amount of red blood cells so that the ratio of red blood cells to serum is 1:10 (for convenience, it is recommended to first release a few drops of red blood cells from the container through a needle onto the edge of the plate, then from there transfer a small a drop of red blood cells into the serum). Next, the red blood cells are mixed with the serum, the plate is gently rocked for 5 minutes, observing the progress of the reaction. After the specified time, 1-2 drops of physiological solution can be added to the reaction mixture to remove possible nonspecific aggregation of red blood cells.
Accounting for results. The presence of red blood cell agglutination means that the donor's blood is incompatible with the recipient's blood and should not be transfused. If after 5 minutes there is no agglutination of red blood cells, this means that the donor’s blood is compatible with the recipient’s blood for group agglutinogens.
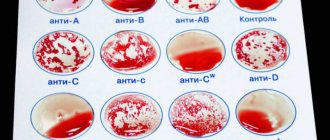
AB0 blood group system
Determination of blood group according to the AB0 system is based on the detection of group-specific antigens 0, A, B and isoimmune antibodies anti-A (α) and anti-B (β) in the serum on the surface of human red blood cells. A unique feature of the AB0 system is the presence in human plasma of natural α and β antibodies to the missing antigen. There are six possible allelic antigen options: 00, A0, AA, B0, BB, AB. Phenotypically, four groups are distinguished: 0(I), A(II), B(III), AB(IV) - hetero- and homozygous variants are combined (in Russia they use alphanumeric designations).
Group affiliation according to the AB0 system
| Agglutinogens | Agglutinins |
| 0 | α and β |
| A | β |
| B | α |
| AB | No |
- 0(I): antigens A and B are absent, antibodies α and β are detected (35 - 40% of the world's population);
- A(II): A antigen and β antibodies present (35%);
- B(III): agglutinogen B and agglutinin α were detected (15 – 20%);
- AB(IV): presence of agglutinogens A and B, absence of agglutinins α and β (5 – 10%).
As you move from west to east of Eurasia, the frequency of detection of antigen A decreases, and antigen B increases. Antigen 0 is rare in Asia, but is widespread in the indigenous peoples of South America, Polynesia and Australia. The reason is epidemics of infectious diseases.
The result of blood typing is recorded in the medical history or in the donor’s card. The transfusiologist indicates the date and signs.
In some cases, mild agglutination of red blood cells is observed during typing. The insufficiently pronounced reaction is explained by the presence of weak variants of antigens A and B. Subgroups A1 and A2 are of greatest clinical importance. Weak variants were first discovered in 1911 by scientists Dungern and Hirszeld. Later in 1930, Landsteiner and Levine proposed the subgroup names A1 and A2. A2 occurs in up to 20% in group A and up to 35% in group AB. Serum from individuals from A2 blood samples may contain anti-A1 antibodies: 2% of cases in group A2 and 30% of cases in A2B. Anti-A1 antibodies are dangerous due to agglutination of group A red blood cells.
Method for determining blood groups A2 and A2B
The detection rate of A2 red blood cells varies significantly depending on the reagents used. We present a comparison of study results using various methods for typing blood groups A2 and A2B.
- Anti-A1 (lectin, phytohemagglutinin). Diagnosticum clearly (+++/++++) agglutinates A1 erythrocytes immediately after mixing with the sample. Does not agglutinate A2 or causes minor agglutination at the fifth minute and later.
- Standard isohemagglutinating serums.
- Anti-A and anti-AB zoliclones.
- Anti-A zolicone is weak.
| Number of samples analyzed | Blood type A (II) | Blood type AB (IV) | ||
| Number of samples analyzed | Group A2 (II) in % | Number of samples analyzed | Group A2B (IV) in % | |
| Anti-A1 (lectin, phytohemagglutinin) | 1592 | 14,7 | 357 | 23,5 |
| Coliclones: anti-A, anti-AB | 3599 | 2,1* | 357 | 7,03* |
| Tsoliclone anti-A - weak | 3587 | 4,5* | 357 | 11,2* |
| Standard isohemagglutinating serums | 1592 | 17,4 | 344 | 34,2 |
Note: * - agglutination is weakly expressed, there are small agglutinates on a pink background.
Anti-A1 (lectin, phytohemagglutinin) provides the greatest accuracy of the study. The test is recommended for identifying A antigen subgroups in children under two years of age. The reason is the physiological immaturity of newborn erythrocytes, which leads to erroneous test results with standard isohemagglutinating sera.
In 1930, Landsteiner and Levine discovered the Aint subtype: an intermediate variant between A1 and A2. This antigen is characteristic of Negroids and reaches 8.5% in people with blood group A. In Caucasians, Aint was observed in only 1% of people with the second blood group. In extremely rare cases, a person lacks all antigens of the ABO system. The Bombay phenotype is caused by the hh genotype. In the absence of antigen H, individuals in this category exhibit anti-A and anti-B antibodies.
Method for determining blood groups
Typing according to the AB0 system is carried out in a direct agglutination reaction using standard hemagglutinating sera or typing reagents with monoclonal antibodies. The use of coliclones is preferable due to their absolute specificity: each reagent does not contain admixtures of other antibodies, ballast proteins and infectious factors.
Algorithm for identifying blood group using hemagglutinating sera
To determine the AB0 blood group by the direct method, two series of standard isohemagglutinating sera are used. Prepare two series of sera from three groups with a titer of 1:32 or higher. Use a separate labeled pipette to collect each serum. Prepare AB(IV) serum for control.
- Provide good lighting and an air temperature of 18 – 25 °C.
- Label the tablet: 0(I) – on the left, A(II) – in the center, B(III) – on the right. At the top center, indicate the donor's last name or the number of the blood being analyzed.
- Apply 1 – 2 drops (approximately 0.1 ml) of serum into the wells in two rows according to the plate labeling.
- Using a pipette or glass rod, place one small drop of the red blood cells being tested next to the drops of serum. The volume of serum should be approximately 10 times the volume of red blood cell fluid.
- Mix the drops in the wells with a stick.
- To speed up the reaction, gently rock the tablet.
- After three minutes, add one drop of NaCl to the wells of the plate in which agglutination has begun. Wait two more minutes.
- After five minutes, evaluate the results of the reaction in the running set. In case of unexpressed agglutination, add one more drop of NaCl.
Reaction results:
- A negative reaction in three wells indicates the absence of antigens on the red blood cells of the test sample. Blood belongs to group 0(I).
- Agglutination in wells with sera 0(I) and B(III) indicates the presence of agglutinogen A and belonging to group A(II).
- The onset of a reaction with sera 0(I) and A(II) indicates the presence of antigen B and group membership B(III).
- The reaction results in all wells indicate the presence of agglutinogens A and B and correspond to the fourth group AB(IV).
In the latter case, you should make sure that there is no nonspecific reaction: apply 2–3 drops of serum corresponding to group AB(IV) to the tablet and add one drop of the analyzed red blood cells. Stir the liquids and evaluate the result after five minutes. The absence of agglutination indicates belonging to group AB(IV), the presence is a sign of a nonspecific reaction. In this case, as well as in case of mild agglutination, repeat the study with other series of sera.
Technique for determining blood group using zoliclones
Monoclonal antibodies to erythrocyte antigens have replaced isohemagglutinating sera. For each typing, one series of anti-A, anti-B, anti-AB reagents is sufficient. The introduction of monoclonal reagents made it possible to significantly simplify and standardize the AB0 typing technique. Here is a brief step-by-step guide to conducting research on a tablet.
- Provide good lighting. Work at room temperature.
- The object of study is erythrocyte-containing media.
- Label the wells of the plate: anti-A, anti-B, anti-AB, or use a plate with a labeled sticker.
- Apply approximately 0.1 ml of the appropriate monoclonal reagent to each of the three labeled wells.
- Add approximately 0.03 ml of red blood cells to be analyzed next to each drop of diagnostic solution.
- Mix the reagent with the red blood cells in the wells using separate individual glass rods.
- Rock the tablet for about three minutes.
- Check for agglutination in the wells.
Usually the reaction is detected within the first seconds after mixing. In this case, weak variants of antigens A and B may cause later agglutination.
blood sample collection
introducing the sample into the well
tablet with added samples
adding zoliclones
tablet with erythrocytes and zoliclones
mixing test samples with reagents
Indirect typing method: algorithm of actions
The determination method is based on the interaction of erythrocytes from pre-typed individuals of groups 0, A, B or a mixture of erythrocytes from several same-group donors with isohemagglutinins α and β in the test serum.
Use dry, clean pipettes when handling each typing reagent. Rinse stir sticks and pipettes in 0.9% NaCl solution.
- Prepare a plate or tablet. Provide good lighting in the room.
- Draw 3–5 ml of blood without stabilizer into a test tube. Let the serum sit for 1.5 - 2 hours at room temperature.
- Wash test red blood cells in 0.9% saline solution. Prepare a 5% suspension.
- Label the sections on the tablet: 0(I), A(II), B(III).
- Place 2 drops (approximately 0.1 ml) of plasma to be analyzed into each of the three wells.
- Add approximately 0.03 ml of test red blood cells to the wells.
- Using separate sticks, mix the typed red blood cells with the serum.
- Rock the tablet gently for 5 minutes.
- Conduct a visual assessment of the results of the agglutination reaction in transmitted light.
Conclusion about group affiliation
| Results of plasma analysis with standard red blood cells | Group affiliation | ||
| 0(I) | A(II) | B(III) | |
| — | + | + | 0(I) |
| — | — | + | A(II) |
| — | + | — | B(III) |
| — | — | — | AB(IV) |
+ — presence of agglutination, — — negative result of the reaction.
- 0(I): reaction in wells A(II), B(III) (antibodies α and β detected).
- A(II): agglutination with erythrocytes B(III) (β agglutinins detected).
- B(III): agglutination in well A(II) (α agglutinins determined).
- AB(IV): no reaction results in all wells (no antibodies detected in plasma).
4.3. Indirect Coombs test
One drop (0.02 ml) of the sediment of thrice washed donor erythrocytes is added to the test tube, for which a small drop of erythrocytes is squeezed out of the pipette and touched to the bottom of the test tube, and 4 drops (0.2 ml) of the recipient's serum are added. The contents of the tubes are mixed by shaking, after which they are placed for 45 minutes in a thermostat at a temperature of +37°C. After the specified time, the red blood cells are washed again three times and a 5% suspension is prepared in physiological solution. Next, add 1 drop (0.05 ml) of red blood cell suspension onto a porcelain plate, add 1 drop (0.05 ml) of antiglobulin serum and mix with a glass rod. The plate is rocked periodically for 5 minutes.
The results are recorded with the naked eye or through a magnifying glass. Agglutination of red blood cells indicates that the blood of the recipient and the donor are incompatible; the absence of agglutination is an indicator of the compatibility of the blood of the donor and recipient.
Preparing for a blood transfusion
In addition to taking a sample for individual compatibility, there are certain rules for transfusion. Under no circumstances should you transfuse cold blood; it must be warmed for about an hour at room temperature. If it is necessary to urgently infuse blood, then all manipulations are done immediately so that the patient’s heart does not stop. It is important to remember that blood transfusions are given at least an hour after eating.
Before starting a blood transfusion, the donor’s blood type must be indicated, and the recipient’s blood type must also be checked. When using blood that has been previously prepared in vials, the metal cap is first torn off, after which the rubber stopper is treated with alcohol.
- Blood transfusion according to blood groups: rules. Universal donors. Blood group compatibility table
It is this cork that is pierced with thick needles, which can have different lengths. The transfusion system is connected to the short needle, and the long one is connected to the catheter.
4.4. Compatibility test using 10% gelatin
Add 1 small drop (0.02 - 0.03) ml of donor erythrocytes into the test tube, for which squeeze out a small drop of erythrocytes from a pipette and touch the bottom of the test tube with it, add 2 drops (0.1 ml) of gelatin and 2 drops (0.1 ml) recipient serum. The contents of the tubes are mixed by shaking, after which they are placed in a water bath for 15 minutes or a thermostat for 30 minutes at a temperature of +46 - 48°C. After the specified time, add 5 - 8 ml of physiological solution to the test tubes and mix the contents by inverting the test tubes 1 - 2 times.
The result is taken into account by viewing the test tubes with the naked eye or through a magnifying glass. Agglutination of red blood cells indicates that the blood of the recipient and the donor are incompatible; the absence of agglutination is an indicator of the compatibility of the blood of the donor and recipient.
Why is a biological blood sample needed?
A biological test for individual compatibility is considered the final stage of preparation before the transfusion itself.
As a rule, 15 ml of blood is infused, after which the patient is observed. If the patient does not develop any symptoms such as shortness of breath, abdominal pain and anxiety, then 10 ml of blood is again injected and this operation is repeated three times. If there is no negative reaction, then the blood transfusion is completed. If the patient still complains about his condition, the blood transfusion is stopped, otherwise transfusion shock may occur. Sometimes a blood transfusion is performed on a patient under anesthesia, but then the pulse rate must be taken into account and blood pressure measured.
4.5. Compatibility test using 33% polyglucin
Add 2 drops (0.1 ml) of recipient serum, 1 drop (0.05) ml of donor erythrocytes into the test tube and add 1 drop (0.1 ml) of 33% polyglucin. The test tube is tilted to a horizontal position, shaking slightly, then slowly rotated so that its contents spread over the walls in a thin layer. This spreading of the contents of the test tube along the walls makes the reaction more pronounced. Contact of erythrocytes with the patient’s serum while rotating the tube should continue for at least 3 minutes. After 3-5 minutes, add 2-3 ml of physiological solution to the test tube and mix the contents by inverting the test tube 2-3 times without shaking.
The result is taken into account by viewing the test tubes with the naked eye or through a magnifying glass.
Agglutination of red blood cells indicates that the blood of the recipient and the donor are incompatible; the absence of agglutination is an indicator of the compatibility of the blood of the donor and recipient. [/td]
What are the dangers of parental blood type and Rh incompatibility for the unborn child?
The fact is that most people have a positive Rh factor, that is, they have a specific protein in their blood. Only one quarter of the world's population does not have this protein. It is this minority that belongs to the carriers of blood with a negative Rh factor.
The Rh factor does not affect the likelihood of conception in any way. But the course of pregnancy greatly depends on the compatibility of the parents’ Rhesus.
Pregnancy proceeds without incident and the child is born full and healthy when the parents have the same Rh factors. There is no threat to the baby even if the mother’s blood is Rh-positive and the father’s is Rh-negative, since in most cases the child inherits the maternal Rh factor.
But if a woman has a negative Rh factor and the baby inherits a positive Rh factor from the father, then incompatibility between the blood of mother and child will arise during pregnancy.
The female body will perceive the fetus as a foreign body, launch its defense mechanism, and the mother’s immune system will begin its fight and reject the unborn baby. This process is called Rhesus conflict.
Modern medicine makes it possible to prevent Rh conflict if parents and doctors know about its possibility in advance, thanks to which the majority of married couples who are incompatible by blood were able to become happy parents.