From the article you will learn the norm of lymphocytes (LYM, LYMPH in a blood test) for adults and children, the reasons for the increase and decrease in cell concentration.
Lymphocytes (LYM, LYMPH) are the main cells of the immune system, which are responsible for all of our internal defenses. If we decipher the word “lymphocytes” itself, it consists of two ancient Greek words – lymph and cytos.
Lymph is a container, and cytos is a cell. Lymphocytes are representatives of white blood cells, which are the main cells of the immune system. From the moment they appear in the bone marrow until they must perform their particularly important function - to stand on our internal genetic purity - a certain period of time passes.
Types of lymphocytes
When the bone marrow releases a young lymphocyte into the bloodstream, it is not yet ready to perform its most delicate functions. Some of the young cells, lymphocytes, go to the thymus, or thymus gland, and there they must undergo maturation or “training.” What does "training" mean? A young lymphocyte must become familiar with the antigens that our body has encountered throughout life, starting from prenatal development. After maturation, thymus-dependent lymphocytes (T-lymphocytes) will begin to recognize antigens, understand that these are genetic strangers and will be ready to carry out their tasks.
T lymphocytes
There are several types of T-lymphocytes.
The first type is killer T-cells. Killers are killers. They are the ones who are sharpened to recognize a genetic stranger and try to kill him right away.
The second type is T-helper cells, or helper cells. It is the T helper cells that will include the following cells that will produce antibodies or protective protein structures for us.
The third type is T-suppressors - those cells that will stop the immune response.
And so, if a genetic alien enters the human body, the killers will be the first to recognize it and will try to kill it immediately. What can act as a genetic stranger? These are viruses, bacteria, parasites, fungi, and cancer cells that can appear every second. Cells of the body in which a failure occurred at the genetic level. If a person has food intolerance, then, unfortunately, they can also turn into genetic strangers and also deplete the lymphocyte component of the immune system.
B lymphocytes
These are also lymphocytes, which, having emerged young from the bone marrow into the bloodstream, rush to the lymph nodes, to the spleen, or to the liver, where they will also mature. The main function of B cells is to produce representatives of humoral immunity, or blood immunity, for us. It is the B cells that will produce antibodies that will fight viruses, bacteria, and other genetic invaders.
NK cells or natural killer cells
This is a group of cells that will primarily be focused on protecting us from cancer. First of all, natural killers will work in antitumor defense and antiparasitic defense. NK cells are the main types of lymphocytes.
general characteristics
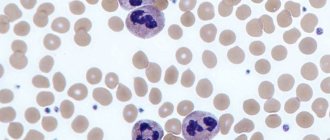
Lymphocytes belong to the leukocyte family. These white cells, whose size does not exceed 10 microns, were discovered a long time ago. Their norm in the blood is 20-40% of the total number of leukocytes. Lymphocytes are cells, most of which are formed in the red bone marrow. They are detected during a wide variety of events occurring in the body. The functions of the smallest cells of the leukocyte family are varied.
First of all, thanks to them, the immune system learns about the penetration of pathogenic elements into the body that have a destructive effect. After receiving such information, natural protective reactions are activated. In addition, lymphocytes form an immune barrier that minimizes the risk of infection.
Under the influence of these cells, foreign tissue is rejected. In particular, this happens during transplantation. Lymphocytes also play an important role in the fight against malignant tissue degeneration in the body. They are always present during cell division, regardless of its nature: natural or pathological.
The norm of lymphocytes in the blood in adults and children
Unfortunately, there are often situations when lymphocytes in the blood can deviate from the norm - increase or decrease. This can happen in various situations. Before we talk about when the number of lymphocytes changes in the blood, we need to talk about how much is their normal component.
If we talk about cell clones, then:
T cells make up 75% of the total number of lymphocytes;
B cells about 15%;
Natural killers are about 10%.
And speaking about the norms of lymphocytes, it must be said that at different periods of a person’s life the norms differ.
Lymphocytes begin to be produced by the bone marrow already in utero, and this process continues throughout life. The lifespan of these cells also varies - from several months to sixty years. And these will all be lymphocytes.
- Up to 1 year, the content of lymphocytes in the blood of the total number of all cells is 45-70%.
- From 1-2 years, the concentration of lymphocytes should decrease - somewhere around 39-60%.
- From 2-4 then somewhere from 33-50% of all white cells.
- From 4-10 years, already 30-50%.
- From 10-18 years old, then this is already 30-44%.
- In an adult, lymphocytes in the blood should range from 19-37%.
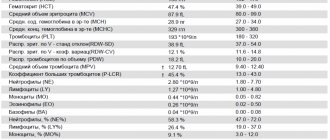
The table shows the values of acceptable norms of lymphocytes (individual subpopulations) in the blood of children and adults.
| Age | Proportion of the total number of lymphocytes, % | Absolute cell count, *106/l |
| CD 3+ (T lymphocytes) | ||
| Up to 3 months | 50 – 75 | 2065 – 6530 |
| Up to 1 year | 40 – 80 | 2275 – 6455 |
| 1 – 2 years | 52 – 83 | 1455 – 5435 |
| 25 years | 61 – 82 | 1600 – 4220 |
| 5 – 15 years | 64 – 77 | 1410 – 2020 |
| Over 15 years old | 63 – 88 | 875 – 2410 |
| CD3+CD4+ (T helper cells) | ||
| Up to 3 months | 38 – 61 | 1450 – 5110 |
| Up to 1 year | 35 – 60 | 1695 – 4620 |
| 1 – 2 years | 30 – 57 | 1010 – 3630 |
| 25 years | 33 – 53 | 910- 2850 |
| 5 – 15 years | 34 – 40 | 720 – 1110 |
| Over 15 years old | 30 – 62 | 540 – 1450 |
| CD3+CD8+ (T-cytotoxic lymphocytes) | ||
| Up to 3 months | 17 – 36 | 660 – 2460 |
| Up to 1 year | 16 – 31 | 710 – 2400 |
| 1 – 2 years | 16 – 39 | 555 – 2240 |
| 25 years | 23 – 37 | 620 – 1900 |
| 5 – 15 years | 26 – 34 | 610 – 930 |
| Over 15 years old | 14 – 38 | 230 – 1230 |
| CD19+ (B lymphocytes) | ||
| Up to 2 years | 17 – 29 | 490 — 1510 |
| 25 years | 20 – 30 | 720 – 1310 |
| 5 – 15 years | 10 – 23 | 290 – 455 |
| Over 15 years old | 5 – 17 | 100 – 475 |
| CD3-CD16+CD56+ (NK cells) | ||
| Up to 1 year | 2 – 15 | 40 – 910 |
| 1 – 2 years | 4 – 18 | 40 – 915 |
| 25 years | 4 – 23 | 95 – 1325 |
| 5 – 15 years | 4 – 25 | 95 – 1330 |
| Over 15 years old | 4 – 27 | 75 – 450 |
| Over 15 years old | 1 – 15 | 20-910 |
The work of resident T cells: do not confuse tourism with emigration
In a normal situation, mouse resident tissue cells hardly move within non-lymphoid tissue and are quite firmly attached by adhesion molecules to the stroma of the organ. When resident macrophages of the same tissue initiate an inflammatory response by secreting cytokines, TRMs become more motile and patrol the nearby epithelium in search of infected cells.
If the inflammatory reaction intensifies, then the cells understand this as a signal for reinforcement: TCM and TEM cells newly arriving from the blood are connected to the work of patrol TRM. These blood cells are much more mobile and move better in the epithelium. Does this mean that it is in the blood that killer T-cells are ready to act among TEM, and CD8+ TRMs perform helper and regulatory functions in the tissue?
On the one hand, T-helpers are more tissue-specific in the spectrum of T-cell receptors, i.e. There is very little overlap between the TCR repertoires of cells taken from different tissues, while cells of the same killer T cell clone are found in different tissues among TEMs []. The range of functions and repertoire of antigen specificity of TRM remains to be studied, but TRM killers definitely have the ability to destroy infected tissue cells. Moreover, in a model of murine polyomavirus infection in brain tissue, the affinity of virus-specific T-cell receptors of resident killer cells is higher than that of virus-specific central memory cells [].
However, the size of the T-cell population depends not only on the specificity of the TCR for infections that previously occurred in a given organ, but also on the homeostatic proliferation of T-cells - the proliferation of more successful cells to fill the organ's capacity according to the number of T-lymphocytes. By using the markers CD28 and CD127 on the cell surface, it is possible to distinguish cells recently and long ago activated through the TCR from those that received only a homeostatic signal to proliferation from the growth factor IL-7. As tissue ages, homeostatic cell proliferation begins to dominate over the proliferation of TCR-activated cells.
NKT cells, a type of liver-resident cell found in other tissues, often function independently of T-cell receptors. They can be activated by NK cell receptors through recognition not of individual antigens, but of general molecular patterns of danger and tissue stress. When activated, CD8+ NKT cells release cytotoxic granules and lyse suspicious tissue cells, for example, single tumor cells and virus-infected cells that express and display stress molecules on the outer membrane. With aging, the tendency of TRM to be activated without the T cell receptor, through NK cell receptors or cytokine signals, can lead to erroneous lysis of tissue cells, insufficient control of chronically infected or degenerating areas of the epithelium.
Pathological manifestations associated with the work of resident T cells include organ-specific autoimmune syndromes and chronic tissue inflammation syndromes. Examples of chronic inflammation maintained by resident T lymphocytes are contact dermatitis and psoriasis, and the mechanism is the release of inflammatory factors IL-17 by resident killer T cells and IL-22 by resident dermal helper T cells. CD8+ effector T killer cells located in the brain are similar in their set of membrane marker molecules to the TRM of the skin, intestines and lungs and are able to push the development of intermittent multiple sclerosis with periodic releases of inflammatory cytokines. It is unclear, however, whether there is a normal TRM population in the brain or whether these are T lymphocytes remaining in the tissue after a neurotropic viral infection [].
The functions of resident memory cells normally—in the absence of infection or chronic inflammation—may include cross-talk
(mutual regulation mainly through the secretion of cytokines and costimulatory molecules) with non-classical, poorly studied lymphoid cells.
They may be mucosal-associated γ/δ T cells, which carry an alternative variant of T-cell receptor assembly, or Innate Lymphoid
(ILCs), which share common features of the epigenetic landscape with T and B lymphocytes, but do not have T-/B- or NK-cell receptors [, ].
Proposed functions of tissue resident T-lymphocytes. Some functions can be performed in interaction with resident macrophages
TRM cells come into contact with antigen-presenting tissue cells—dendritic cells of the skin and resident tissue macrophages. Resident myeloid cells in different tissues are differentiated and slightly similar to each other. For example, marginal zone macrophages of the spleen, liver macrophages, and microglia (brain macrophages) will differ greatly in both morphology and range of functions. In addition to detecting antigens in tissue, resident macrophages are involved in regulating the processes of aging and tissue self-renewal, in particular, they secrete growth factors and cytokines that stimulate the division of tissue stem cells. In adipose tissue, for example, macrophages stimulate the differentiation of new fat cells, but when they enter an activated M1 state, they trigger inflammation and, instead of differentiating, cause existing fat cells to enlarge and swell. Concomitant changes in adipose tissue metabolism lead to the accumulation of fat mass and in recent years have been associated with the mechanisms of development of obesity and type II diabetes. In the skin, cytokines released by macrophages and resident γ/δ T cells stimulate stem cell division during epidermal and hair follicle stem cell regeneration [, ]. It can be assumed that helper TRM cells, when patrolling the epithelium and forming contacts with tissue macrophages, can modulate the spectrum and volume of growth factors secreted by the latter for stem cells, inflammatory cytokines and epithelial remodeling factors - and thereby participate in tissue renewal.
Causes of increased lymphocytes in the blood in adults
What does it mean? The causes of elevated lymphocytes in the blood in women and men may be different, but there are several types of diseases that most often lead to this phenomenon:
- infectious diseases;
- bacterial infections;
- autoimmune diseases;
- acute allergies and anaphylactic shock are possible;
- the appearance and growth of malignant and benign tumors and neoplasms;
- especially pronounced lymphocytosis will be in tests for diseases that can be suffered only once (measles, rubella, chickenpox, mononucleosis, etc.);
- autoimmune processes.
How to distinguish resident tissue cells from admixtures of blood cells?
Resident T cells can be correctly, but inconveniently, determined each time by the ability of an individual cell to migrate to lymph nodes, so it is necessary to compile a list of characteristic features that can be used to identify membership in this subpopulation. Resident T lymphocytes in tissues that are the body's natural barriers (for example, in the lungs and small intestinal mucosa) are a bit like classical effector blood cells: they express the activated cell marker CD69, and the expression is stable throughout life during adulthood and aging and is characteristic of all non-lymphoid tissues. But in addition, CD69 colocalizes with the marker CD103, which denotes a group of adhesion molecules - integrins, which promote the attachment of resident T cells to the epithelium and to fibroblasts in the submucosa of the selected organ. For effector T cells in secondary lymphoid organs, the expression of CD103 integrins is completely uncharacteristic: TEM cells constantly maintain a motile phenotype.
The map compiled by Donna Farber's team has a major flaw: it is unclear how cleanly T-lymphocytes can be isolated from an organ, and what proportion of the analyzed cells are actually blood T-lymphocytes from capillaries inside the organ.
The issue of contamination by blood cells is especially acute for the lungs - it is no coincidence that the subpopulation composition of T cells in the lungs is unexpectedly similar to T cells in the blood and lymph nodes. The issue of blood cell contamination was elegantly solved for mouse T cells: experimental animals were infected with lymphocytic choriomeningitis virus after transplantation of a transgenic P14 T cell clone specific for this virus. As a result, during infection, the majority of circulating cells were represented by the virus-specific P14 clone, and its presence in tissues could be detected using fluorescent antibodies to TCR P14. Mice were injected into the blood with an anti-CD8 antibody to a marker of T-killer cells; it quickly spread through the bloodstream and bound to all T-killer cells in the blood (but not in tissues). By microscopy of organ sections, it was easy to distinguish resident killer TRMs from cells only recently released from the blood into the organ, labeled with anti-CD8 antibody []. The number of resident cells calculated by this method was 70 times higher than the number determined by flow cytometry; a difference of less than twofold was observed only for resident cells of the lymph nodes and spleen. It turns out that standard methods for isolating lymphocytes from organs are poorly suited for the analysis of killer resident cells and significantly underestimate the size of the population.
Lymphocytes are higher than normal in children
In children 4-5 days and 4-5 years of age, physiological lymphocytosis is observed in the blood, which does not require treatment. The child’s condition remains completely normal, the lymph nodes do not enlarge. This situation is due to the restructuring of the child’s hematopoietic system.
However, an increased lymphocyte count in children may be caused by:
- Leukemia;
- Bronchial asthma;
- Infection: influenza, ARVI, sore throat and others;
- Purulent-inflammatory processes;
- Viral diseases: lichen, whooping cough, malaria, varicella (chickenpox), measles, viral hepatitis and others.
Lymphocytes can also be elevated during other diseases, with various individual characteristics of the body. The exact reasons can only be determined after a full examination.
References
- Khaitov, R.M. Allergology and immunology: national guide / ed. R.M. Khaitova, N.I. Ilyina. - M.: GEOTAR-Media, 2009. - 656 p.
- Mayorov, R.V., Nusinov, E.V. Methods for assessing immune status. Recommendations for students, 2012.
- Histology (introduction to pathology" / edited by E.G. Ulumbekov, Yu.A. Chelyshev. - M.: GEOTAR-Media, 1997. - P. 527-545.
- Serrano-Villar, S., Deeks, S. CD4/CD8 ratio: an emerging biomarker for HIV. Lancet HIV, 2015. - Vol. 2. - P. 76-7.
- Castilho, J., Shepherd, B. CD4+/CD8+ ratio, age, and risk of serious noncommunicable diseases in HIV infected adults on antiretroviral therapy. AIDS, 2021. - Vol. 30. - P. 899–908.
- Leach, M., Drummond, M., Doig, A. Practical Flow Cytometry in Haematology Diagnosis Hardcover. WILEY-BLACKWELL, 2013.
- Tietz Clinical guide to laboratory tests. 4th ed. Ed. Wu ANB - USA: WB Sounders Company, 2006. - 1798 p.
What to do when there are high lymphocytes in a blood test
If lymphocytes are elevated, what should you do in this case? There can be only one answer: to identify and eliminate the cause of this condition. When lymphocytes are elevated, treatment should not be aimed at reducing their level, but at the disease itself.
Depending on the disease, therapy takes from several days to several months and usually helps stabilize the level of lymphocytes. For example, for most infectious processes, anti-inflammatory, antipyretic, antiviral drugs, as well as antibiotics are prescribed. The course of treatment for myeloma and leukemia is very unique and often requires chemotherapy and bone marrow transplantation.
Features of lymphocytosis
The term indicates disturbances in the functioning of the female body, the activity of the immune system to combat intrusions:
- bacteria, viruses;
- protozoa, parasites.
Other reasons for an increased concentration of lymphocytes include their damage or death due to:
- burn disease;
- tissue necrosis;
- inflammatory processes;
- allergies;
- autoimmune diseases;
- oncological pathologies.
Cells reach problem areas, but with further progression of the disease they begin to die en masse.
The chronic form of lymphocytosis is the result of diseases of the blood or hematopoietic system, lymphatic department. Taurus do not fully mature and cannot fully perform the functions assigned to them. To normalize the balance, the bone marrow produces even more lymphocytes, but the benefits from the increased work are minimal.
The accumulation of protective cells may be due to problems with their utilization in the spleen. Doctors believe that it is not deviations in tests that are of greatest importance, but the sources of their occurrence. Dysfunction of the immune system leads to the development of chronic infections, autoimmune diseases with attacks on one’s own cells, the formation and further proliferation of atypical structural units.
Life cycle of a T lymphocyte
Each T cell, after assembling a T-cell receptor, is tested for the functionality of the randomly assembled receptor (positive selection) and the lack of specificity for the body's own antigens (negative selection), that is, for the absence of an obvious autoimmune threat. The stages of selection occur in the thymus gland, thymus; in this case, more than 90% of precursor cells die, failing to correctly assemble the receptor or undergo selective selection. Surviving T cells proliferate and exit the thymus into the bloodstream - these are naïve T cells that have not encountered the antigen. The naive T cell circulates through the blood and periodically enters the lymph nodes, where in the T-cell zone it contacts specialized antigen-presenting cells.
After meeting the antigen in the lymph node, the T cell acquires the ability to divide again - it becomes the precursor of memory T cells (TSCM, stem cell memory T cells). Among the clone of its descendants, central memory cells (TCM), short-lived effector cells that carry out the immune response (SLEC or TEMRA cells), and effector memory precursor cells TEM, which in turn produce TEMRA when dividing, appear [3]. All these cells leave the lymph node and travel through the blood. Effector cells can then leave the bloodstream to carry out an immune response in the peripheral tissue of the organ where the pathogen resides. What then - another journey through the blood and lymph nodes?
Figure 1. Effector T cell emigration into tissue during viral infection. Inflammatory signals from infected epithelial cells with the participation of resident cells are transmitted to the vascular endothelium; endothelial cells attract effector T cells with chemokines CXCL9, CXCL10. Rolling: When moving along a postcapillary venule in tissue, the effector cell slows down, forming temporary contacts between E-selectins and P-selectins on endothelial cells. Arrest: The effector cell adheres tightly to the endothelium when LFA-1 and other alpha integrins interact with ICAM-1/VCAM-1/MAdCAM-1 (on the endothelium). Transmigration: The effector T cell binds endothelial JAM-1 with molecules PECAM, CD99, LFA-1 and penetrates through endothelial cells into the submucosa. Figure from [3].
The process of leukocyte transmigration.
The cells of the stroma, that is, the base of the lymph node, secrete signaling substances in order to call the T cell to the lymph node - chemokines. Lymph node chemokines are recognized by homing receptors CCR7 and CD62L. But effector cells lack both of these receptors. Because of this, it has long been a mystery how effector cells can get from peripheral tissue back to the secondary lymphoid organs - the spleen and lymph nodes.
At the same time, evidence began to accumulate of differences in membrane marker repertoires and transcriptional profiles between memory T cells in the blood (TEM) and memory T cells in other organs, which did not fit into the concept of constant migration of T cells between tissues and blood . It was decided to isolate a new subpopulation: resident memory cells that inhabit a specific organ and do not recycle - TRM cells [4].
Figure 2. Scheme of the transition of descendants of activated T lymphocytes between populations. Figure from [14].