Why does the body need eosinophils?
Each cell of our body plays its role. Now we will talk about eosinophils.
What are eosinophils?
Everyone knows that in our body there are erythrocytes (red blood cells) and leukocytes (white blood cells).
But few people know that leukocytes are also divided into:
- cells containing granules in the cytoplasm. These include basophils, neutrophils, eosinophils;
- cells that do not contain granules in the cytoplasm. Representatives of this group are monocytes and lymphocytes.
Thus, eosinophils are a type of leukocyte that contains granules.
What kind of granules are these? These granules are found in the cytoplasm. Therefore, when staining cells, they are the ones that give eosinophils their bright red color. What do eosinophil granules contain? Eosinophils contain a number of substances that ensure the performance of their functions. These include:
- main basic protein - promotes the destruction of parasites due to its toxic effect;
- cationic protein of eosinophils - also has toxic activity towards helminths, suppresses the synthesis of antibodies, helps in interaction with other cells;
- eosinophilic peroxidase – promotes the oxidation of substances, which results in the formation of reactive oxygen species. They, in turn, are capable of exposing the cell to death;
- eosinophil neurotoxin - exhibits its activity against viruses, activating cells of the immune system to develop an inflammatory response.
In addition to the fact that eosinophils have specific granules, these cells are capable of producing various signaling molecules. They are called cytokines. They ensure the functioning of cytokines at the site of inflammation and participation in the activation of the immune system.
Place of synthesis
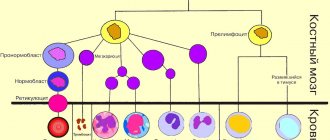
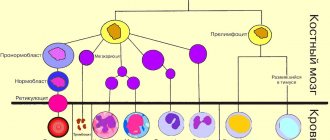
All blood cells mature in the bone marrow. There, from the universal precursor cell, eosinophil maturation occurs (Figure 1).
Fig.1. Scheme of eosinophil maturation.
A mature cell, a segmented eosinophil, enters the bloodstream. If young forms are detected in the blood, this may indicate excessive destruction of eosinophils or the receipt of a large number of signals in the bone marrow to stimulate the formation of these cells.
A signal came to the bone marrow about the need for the synthesis of eosinophils, and after 4 days these cells are waiting for their turn to enter the bloodstream.
Eosinophils circulate in the blood for only a few hours, after which they go into the tissues and stand guard over order. They remain in tissues for about 10–12 days.
A small number of eosinophils are found in tissues that border the environment, providing protection to our body.
What functions do eosinophils perform?
It has already been said before what effects eosinophils can perform due to specific granules in the cytoplasm. But in order for eosinophils to be activated, that is, to release the contents of the granules, some kind of signal is required. Basically, this signal is the interaction of activators with receptors on the surface of eosinophils.
The activator can be antibodies of classes E and G, the complement system activated by helminth components. In addition to directly interacting with the surface of eosinophils, mast cells, for example, can produce chemotaxis factor, a compound that attracts eosinophils to that site.
Based on this, the functions of eosinophils include:
- participation in an allergic reaction. During an allergic reaction, histamine is released from basophils and mast cells, which determines the clinical symptoms of hypersensitivity. Eosinophils migrate to this area and promote the breakdown of histamine;
- toxic effect. This biological effect can manifest itself in relation to helminths, pathogenic agents, etc.;
- possessing phagocytic activity, they are able to destroy pathological cells, but neutrophils have a higher ability;
- Due to the formation of reactive oxygen species, they exhibit their bactericidal effect.
The main thing to remember is that eosinophils are involved in allergic reactions and the fight against helminths.
The normal level of eosinophils in the blood of a child
As mentioned earlier, eosinophils do not stay in the bloodstream for a long time. Therefore, healthy children should not have many eosinophils.
The numerical values of the norm depend on the method used to determine the number of cells. In old laboratories, the leukocyte formula is calculated manually, the result is given only in relative values, that is, in %.
Normally, in children under 4 years of age, the relative number of eosinophils should not exceed 7%. Over this age, the norm is the same as for adults - no more than 5%.
In modern laboratories, cells are most often counted automatically on a hematology analyzer, and only in exceptional cases are they counted manually. When counting cells on the analyzer, the result can be given in the form of relative and absolute values.
The absolute number of eosinophils reflects their exact number per liter of blood.
The absolute values of normal eosinophils are presented in the table.
Table. The norm of eosinophils in the blood of children.
| Age | Absolute quantities |
| Up to a year | 0.05 - 0.4 *10*9/l |
| One year - six years | 0.02 - 0.3 *10*9/l |
| More than six years | 0.02 - 0.5 *10*9/l |
Data with normal values are given for informational purposes only; you should not decipher the results of the analysis yourself!
Norms of eosinophils in children
To find out whether a child’s eosinophils are elevated or not, you need to know what the norm is. Its indicators vary depending on the age of the child. Since eosinophils are often recorded as a percentage, the figures for different age groups are as follows:
- from birth to two weeks – 1–6%;
- from two weeks of age to one year – 1–5%;
- 1–2 years – 1–7%;
- 2–4 years – 1–6%;
- 5–18 years – 1–5%.
As can be seen, eosinophils may be present in the blood in small quantities. This is normal and does not require correction.
Indications for determining the level of eosinophils in the blood
Since eosinophils play an important role in allergic reactions and the fight against parasites, it is advisable to determine the level of these cells in the blood if these processes are suspected.
That is, the main indications for determining the level of eosinophils in a child’s blood may be:
- after eating some food product, itching began, dermatitis appeared on the skin, damage to the respiratory tract (coughing, sneezing, swelling of the larynx), and so on;
- headaches, poor perseverance of the child, insomnia may indicate the presence of parasites;
- low body weight with increased appetite may also indicate helminthiasis;
- the process of digesting food is disrupted, accompanied by changes in stool, vomiting;
- stomach ache;
- signs of deficiency of essential nutrients, despite adequate feeding of the child;
- Body temperature may rise.
If your child is crying, then something is bothering him, but he cannot tell you about it. Therefore, it is extremely important to understand what is happening to it and prevent the development of serious complications.
In addition to food allergies, it is possible to develop hypersensitivity to dust, animal hair, pollen, and even medications.
Eosinophilic gastrointestinal diseases in children. It's time to make a diagnosis
In the modern classification of food allergies, a group of eosinophilic gastrointestinal diseases is separately identified, in particular, eosinophilic gastroenterocolitis and eosinophilic esophagitis (EoE) (Table 1) [1, 2]. The pathogenesis of these types of food allergies is associated with mixed – IgE- and non-IgE-mediated – development mechanisms and infiltration of eosinophils in the mucous membrane of the esophagus, stomach and intestines [1–4].
As you know, food allergies are characterized by any combination of symptoms from the skin, gastrointestinal tract (GIT) and respiratory tract. Among the polymorphism of clinical manifestations of food allergy, skin lesions occur in approximately 80% of cases, while eosinophilic gastrointestinal diseases are much less common [1–5]. In addition, there is an additional underdiagnosis of EoE and allergic (eosinophilic) gastroenteritis. There are several reasons for this. Thus, if in the 90s EoE was considered a rare disease (1–2 publications in the PubMed database), today this nosology is widely recognized throughout the world in children and adults [1–3]. The development of clear clinical diagnostic criteria and basic approaches to the treatment of EoE played an invaluable role in this issue [6]. In 2011, an international group of experts proposed the following conceptual definition of the disease: “Eosinophilic esophagitis is a chronic, immune-mediated disease, histologically characterized by pronounced eosinophilic infiltration of the esophageal mucosa and clinically manifested by esophageal dysfunction (dysphagia)” [6]. Thus, it becomes clear that the most important reason for underdiagnosis of eosinophilic gastrointestinal diseases is the lack of an integrated approach, since confirmation of the clinical diagnosis requires the participation of a pathologist and examination of biopsy samples of the mucous membrane of the esophagus and stomach after esophagogastroduodenoscopy (EGDS).
Another diagnostic difficulty for practicing physicians is the timely correct differential diagnosis of gastrointestinal food allergy with other gastrointestinal pathologies. This problem is indicated by the presence of ambiguous symptoms and the absence of a typical clinical picture: nausea, vomiting, abdominal pain, diarrhea, blood in feces, etc., are known to be characteristic of a very wide range of diseases [1–6].
However, recently there have been reports that will allow doctors (primarily pediatricians, gastroenterologists and even neurologists) to highly likely suspect eosinophilic gastrointestinal diseases in a child based on anamnesis and classical tissue biopsy data. Thus, the researchers suggest that doctors take into account 4 clinical features, which together indicate a high likelihood of EoE without the need for a biopsy in the future: these are young children, more often male (60%), suffering from dysphagia (98%), atopy (69%) and food allergy (43%) compared to controls [7]. Four endoscopic criteria for EoE - rings (78%), grooves (86%) and plaques (47%) in the absence of hiatal hernia, together will also help the doctor independently distinguish EoE from gastroesophageal reflux disease (GERD). Some patients with EoE have normal endoscopic findings (4%) [6, 7].
Another recent conclusion by the famous scientist J. Spergel et al. also helps to improve understanding of this nosology [8]. Thus, when observing the dynamics of children with food allergies to milk, eggs, wheat and soy (n = 425), a total of 17 of them subsequently developed EoE. A food was considered a cause of EoE if symptoms improved when it was removed from the diet, and if reintroduction led to their resumption. As before, the most common triggers of EoE in this subgroup of children were milk, eggs, wheat, and soy. In addition, 94% of them suffered from various allergic diseases. After 2 to 4 years, some patients who have outgrown food allergies may develop EoE as a reaction to the same allergenic foods, the authors emphasize.
It is important for allergists to know that approximately 10–15% of patients who receive specific oral immunotherapy (sublingually, in tablets) for food allergies or hay fever may also develop esophagitis [8]. With seasonal pollen allergy, in 20% of patients, clinical symptoms of worsening allergic rhinitis were accompanied by exacerbation of EoE [9].
Assessing a delayed-type allergic reaction requires a careful differentiated approach and a properly conducted examination of the patient.
Eosinophilic esophagitis
Epidemiology
EoE can be diagnosed at any age. Its prevalence has been studied almost all over the world, including isolated publications from African countries [6, 7]. In Western countries, it is estimated to be ~4.4–9.5 cases per 100,000 people [7]. There is very little such data in the Russian literature [10, 11].
The disease is more common among city residents compared to rural areas; with a higher prevalence in cold and arid zones than in tropical climates [11]. Among patients with an established diagnosis of EoE, males predominate (76% of adults and 66% of children) [6, 7]. Familial clusters of EoE have been described, although the exact location of the loci is unknown. A high prevalence of EoE among children suffering from connective tissue disorders has recently been reported.
Clinic
Essentially, EoE provides clinicians with new explanations for previously misunderstood gastrointestinal symptoms such as dysphagia, heartburn, chest pain, vomiting, and abdominal pain in children and adults. Of course, there are certain differences in the clinical manifestations of EoE depending on age (Table 2) [12, 13]. Thus, typical gastrointestinal symptoms of the upper gastrointestinal tract appear mainly in childhood or after 30–40 years. A close relationship has been proven between EoE and atopic diseases (bronchial asthma, allergic rhinitis and atopic dermatitis). Most patients with EoE had food allergies in childhood and often had positive skin or serological tests for foods, although this relationship is less pronounced in adults [12].
Newborns and children of the first 3 years of life (usually males and those suffering from various atopic diseases) are characterized by a variety of nonspecific complaints: frequent refusal to eat, regurgitation, vomiting, abdominal pain.
Older children report a “feeling of a foreign body in the throat”; itching, sore throat; they hold food in their mouth for 15 minutes before swallowing; They chew food slowly and need a lot of water to swallow food. Often such children refuse food (spit out food; limit food intake on their own or are very picky about food; they may have a fear of choking, problems with falling asleep) [13]. These children also experience symptoms of GERD with varying frequencies: heartburn and reflux (5–82%), vomiting (5–68%), abdominal pain (8–100%).
In adolescents and adults, EoE is represented by typical GERD-like symptoms (vomiting, nausea, abdominal pain, heartburn and regurgitation) [6, 12, 13]. The incidence of dysphagia when eating hard foods (29–100%) and the feeling of food pressure (25–100%) and chest pain tend to increase with age [1–4]. Such patients quite often note changes in their chewing habits (there is a need to chew for a long time and drink plenty of water to complete the swallow) [1–5]. Pain behind the sternum can occur spontaneously or after consuming alcohol, acidic foods, as well as dry, rough foods and fast meals. Finally, some patients with EoE complain of atypical symptoms of GERD (bronchial asthma attacks, hoarseness, cough, rhinosinusitis, sleep disturbances (10–25%) [6].
Episodes of food herniation into the esophagus (when the contents of the food bolus, despite the additional efforts made by the patient, remain in the esophagus), esophageal strictures, and “narrow esophagus” are the main complications of EoE [6, 7, 13]. EoE can also be detected during spontaneous perforation (transmural/intramural or deep lacerations during endoscopy). Such complications are extremely rare.
A doctor's examination and standard laboratory tests, as a rule, do not reveal any significant abnormalities, with the exception of mild eosinophilia in the peripheral blood and an increase in the level of total IgE (in 50% and 70% of patients with EoE, respectively) [6, 7].
Regardless of age, any patient with GERD-like symptoms that are difficult to respond to pharmacotherapy or surgical treatment should exclude EoE [6]. Heartburn refractory to traditional therapy in children and adults is another cause of undiagnosed EoE.
Thus, in adolescents and adults, the predominant symptoms of EoE are dysphagia and difficulty swallowing.
Dysphagia is a rather alarming symptom when it accompanies reflux, and necessarily requires an endoscopy in all cases. Patients describe dysphagia as a feeling of difficulty swallowing solid and/or liquid food, “sticking out in the chest” (Table 3) [6, 7, 14].
As you know, swallowing occurs in three stages: in the mouth, pharynx and esophagus. Oropharyngeal dysphagia is observed with neuromuscular disorders (stroke, parkinsonism), as well as dry mucous membranes caused by drugs or radiation therapy. In addition to EoE, esophageal dysphagia is usually associated with anatomical defects of the esophagus (including GERD) and motility disorders (eg, cardia). In case of structural disorders of the esophagus (inflammatory and oncological diseases), dysphagia appears after consuming only solid food, while in patients with impaired motility it is observed in response to liquid and solid foods (Table 4) [15]. However, in patients with dysphagia, the most common cause is EoE.
Typical clinical symptoms of EoE - dysphagia, heartburn and chest pain - are very similar to many other diseases (primarily GERD). According to the recommendations of experts, to confirm the diagnosis of EoE, such patients should be prescribed a repeat morphological assessment of esophageal biopsies after a 2-week course of treatment with proton pump inhibitors [6].
Endoscopy for EoE
Upper endoscopy is the first diagnostic step in the evaluation of all patients with dysphagia. However, there are no pathognomonic endoscopic signs or changes in the esophageal mucosa specific to EoE. And the definition of the disease itself does not include typical or any features of the endoscopic picture. If EoE is suspected, experts advise always performing a biopsy, even if the mucosa is normal or the patient has other potential causes of dysphagia (stenosis, etc.) [6, 7, 15].
Among the most common endoscopic signs of EoE are fixed concentric rings (tracheal esophagus, or “trachealization”), movable concentric rings (cat esophagus, “cat sulcus,” or “felinization”), vulnerability (tears) of the mucous membrane (tissue paper type mucosa). "") and its edema [6, 7, 15, 16]. The formation of rings has been associated with edema, inflammation, and possible tissue fibrosis. Chronic inflammation promotes the formation of scars and strictures, which lead to permanent esophageal stenosis [17]. In fact, impaired swallowing and the presence of strictures are the main complications of EoE. In the 90s, the cause of benign esophageal strictures in up to 80% of cases was peptic ulcer and GERD, but in the last decade their number has sharply decreased due to the widespread use of proton pump inhibitors.
Esophageal strictures are classified as simple or complex depending on their diameter and anatomical abnormalities. Strictures are also found in cases of hiatal hernia, esophageal diverticula, or tracheoesophageal fistula [15–17]. “Narrow esophagus” differs from strictures in the significant extent of narrowing of the lumen, affecting most of the esophagus [17]. The risk of developing esophageal strictures is associated with underdiagnosis of the disease: in particular, in young patients, the period from the onset of the first symptoms to the diagnosis of EoE is on average 10 years [17]. In addition, over the past 20 years, EoE has become one of the leading causes of narrowing the caliber of the esophagus (other causes are prolonged intubation with a nasogastric tube, radiation esophagitis, caustic injuries, Barrett's esophagitis, bullous skin diseases, congenital stenosis of the esophagus, intramural pseudotuberculosis of the esophagus, etc. .).
Other pathomorphological signs of EoE: eosinophilic microabscesses (accumulation of > 4 eosinophils within the epithelial layer), infiltration of eosinophils in the superficial layers of the epithelium; Less common are hyperplasia of the basal layer of the epithelium, intercellular edema, an increase in the number and elongation of the papillae of the lamina propria, its fibrosis/sclerosis, an increase in the number of mast cells, and the accumulation of T- and B-lymphocytes [6, 15–17]. Fibrinous exudate (granular mucosal surface, whitish papules of various sizes) is formed due to inflammation of the esophageal epithelium and represents eosinophilic abscesses, which are mistaken for esophageal candidiasis. Erosive and ulcerative lesions of the esophageal mucosa, as well as neutrophil infiltration, are not typical for EoE, unless such a patient simultaneously suffers from other diseases (GERD, infectious esophagitis, etc.). At the same time, in severe cases of the disease, ulcerative lesions and vomiting may appear during the endoscopic procedure itself.
According to the new classification, the endoscopic picture of EoE includes 4 main ones (rings, grooves, exudate, edema), as well as additional parameters (narrowing of the esophagus, moving rings, stricture and vulnerability of its mucous membrane) (Table 5) [16]. Thus, during the course of EoE, two variants are distinguished: those occurring with inflammation and fibrotic changes. Thus, white exudate, edema and linear grooves, as well as the normal diameter of the esophagus, represent endoscopic elements of acute inflammation. The characteristic endoscopic features of fibrous inflammation in EoE are rings, strictures, and tissue paper-type mucosa. Most patients with EoE exhibit a combination of inflammatory and fibrotic changes [6, 16, 17].
When studying the predictors of the development of EoE based on the above endoscopic data, it was found that the inflammatory phenotype is more typical for young patients and is less often accompanied by dysphagia, a feeling of compression by food and dilatation of the esophagus (p < 0.001) [18]. On the contrary, the probability of developing the fibrostenotic phenotype of EoE significantly increased more than twice every 10 years. This association most likely indicates the natural course of EoE: progression of the disease from inflammatory to fibrostenotic processes. At the same time, in 90% of children suffering from EoE, subepithelial fibrosis was detected, and deep tissue biopsy revealed eosinophilic infiltration of the lamina propria [18].
However, even such endoscopic signs of EoE as esophageal rings, strictures, linear grooves and white exudate are not specific, since they are also characteristic of other diseases of the esophagus and often mislead the endoscopist [6, 15].
History and evidence of dysphagia are clear indications for biopsy sampling even in cases of normal esophageal endoscopy [1–4].
The main histological diagnostic criterion for EoE is intraepithelial eosinophilic infiltration with the number of eosinophils ≥ 15 in the field of view of a high-resolution microscope (×400) [6, 7]. As a rule, eosinophils are practically absent from the tissue of the upper gastrointestinal tract. Significantly pronounced intraepithelial infiltration with eosinophils is detected in approximately 1/3 of patients with visually unchanged esophageal mucosa [6]. In 32% of the examined children, a normal esophagus was endoscopically determined, despite pronounced tissue infiltration with eosinophils (the average number of eosinophils in the proximal and distal parts of the esophagus was 23.3 ± 10.5 and 38.7 ± 13.3, respectively) [6].
At the same time, such a classic histological sign of EoE as esophageal eosinophilia is also not pathognomonic only for this nosology.
Eosinophilia of esophageal tissue
According to the recommendations, an eosinophil count > 15 per visual field is considered the minimum threshold for diagnosing EoE in children and adults [6, 7]. In a small group of patients, <15 eosinophils per field of view may be detected, but other features (microabscesses, eosinophils located in the superficial layers of the esophagus, extracellular eosinophil granules, basal cell hyperplasia, enlarged intercellular space, fibrosis, etc.) indicate eosinophilic inflammation [13–19].
If eosinophilia is detected in the esophageal tissue, it is necessary to exclude other possible causes (Table 6) [7].
Required number of biopsies
The focal nature of inflammation inherent in EoE complicates the histological part of the diagnosis of the disease. This requires the endoscopist to perform proper biopsy sampling and the pathologist to carefully search for morphological changes in the biopsy specimens. Moreover, the biopsy should be performed regardless of the endoscopic picture (it may be normal).
Consensus recommends 2 to 4 biopsies from the proximal and distal esophagus, respectively [6]. In a study by N. Gonsalves et al. Obtaining three biopsies from different places in the esophagus made it possible to correctly diagnose EoE in almost 97% of cases [19]. According to other data, 100% diagnostic sensitivity in adults can be achieved by examining at least 5 biopsies: from the distal part (5 cm above the gastroesophageal junction); the middle of the esophagus (10 cm above the gastroesophageal junction) and the proximal part (5 cm below the upper esophageal sphincter) [15].
However, morphological assessment of five biopsies may not be accurate enough, since they constitute less than 0.03% of the total surface area of the esophagus [20]. In addition, the density of eosinophil infiltration of esophageal tissue is heterogeneous, since EoE is a focal disease [6–13]. When studying the correlation of eosinophil levels with endoscopic data, J. Salek et al. found that the largest number of eosinophils was contained in biopsies obtained from exudates and furrows; rings without grooves or plaques were not characterized by a significant increase in eosinophil density [20].
The new US guidelines also include a new phenotype of EoE: patients who “have esophageal eosinophilia and respond positively to proton pump inhibitor (PPI) treatment by reducing the number of eosinophils in esophageal tissue” [7]. Such patients, as a rule, have typical symptoms of EoE, and if GERD is excluded, they have a positive clinical and morphological response to an 8-week course of treatment with PPIs in high doses (20–40 mg, similar to the treatment regimen for erosive esophagitis). Moreover, this approach also helps to correctly identify patients who simultaneously suffer from EoE and GERD (pH monitoring does not always allow them to be accurately distinguished) and to identify other causes of esophageal eosinophilia [6, 7]. The pathogenesis of the new EoE phenotype is associated with disruption of the epithelial barrier and further activation of immune mechanisms. Omeprazole has been shown to affect the activation of eosinophils (in particular, the expression of eotaxin-3) [7]. At the same time, PPIs reduce the level of esophageal eosinophils in the absence of reflux, and clinical, endoscopic and histological data in patients who are sensitive and do not respond to PPI therapy may even be similar [21].
Since the symptoms of EoE and GERD largely overlap, the differential diagnosis of these diseases presents certain difficulties. To do this, along with characteristic clinical symptoms, the doctor takes into account pathomorphological features (with GERD, there is infiltration of the distal mucous membrane of the esophagus by neutrophils, balloon degeneration of epithelial cells, the formation of erosions and ulcers on the surface of the epithelium; eosinophils are located in the middle layers of the epithelium and do not penetrate, as with EoE, on the surface of the mucous membrane, the number of eosinophils is usually not > 10 per field of view, etc.) (Table 7) [6, 7].
By analogy with EoE, with GERD, pronounced infiltration of esophageal tissue by eosinophils is sometimes also found. If PPI treatment is ineffective, such patients should be ruled out for EoE. They undergo a repeat histological examination of the esophageal tissue after PPI treatment. The development of GERD is most strongly associated with a risk factor such as hiatal hernia.
The American Society of Gastrointestinal Endoscopy recommends that if eosinophilia is detected in the esophagus, eosinophilic gastroenteritis, Crohn's disease, and achalasia should also be excluded. For this purpose, it is advisable to take biopsy samples from the antrum of the stomach and duodenum (especially in children).
Other methods used in the diagnosis of EoE are summarized below.
When examined with barium, some patients with EoE reveal irregularities in the esophagus, as well as a narrowing of its caliber. The use of endoscopic ultrasonography makes it possible to assess thickening of the mucous membrane and muscle membranes in EoE, but the clinical significance of this parameter is unclear. Endoscopic ultrasonography may also help rule out other causes of stricture if stenosis is present. Esophageal manometry can reveal abnormal peristalsis, but such esophageal motility disorders make it virtually impossible to distinguish between EoE and GERD in adult patients. Planimetry technology (EndoFLIP™) uses a catheter with multiple resistance electrodes to assess esophageal compliance. Such an instrument also makes it possible to quite accurately identify narrowing of the esophagus and localized strictures.
Endoscopy is indicated for all patients suffering from dysphagia to identify its cause and exclude malignant and precancerous tissue changes, as well as the need for therapy, including dilatation. Most often, obtaining mucosal biopsies in combination with dilatation does not pose any additional risks for tissue perforation [7].
In children, it is also important to perform a biopsy of the stomach and duodenum. In adults, if there are any endoscopic changes or symptoms in the abdominal cavity, stomach or intestines, tissue biopsies from these areas should be examined.
Eosinophilic gastritis
Eosinophilic gastritis is determined by such a histological criterion as an increase in the level of eosinophils in the stomach. Recently, H. Ko et al. described the clinical, endoscopic and histopathological features in 30 children (mean age 7.5 years), identifying the so-called histological eosinophilic gastritis, a condition in which gastric tissue contains ≥ 70 eosinophils per field of view (×400) [22]. Symptoms and endoscopic features varied greatly between patients, but the history of atopy and food allergy predominated in all. A total of 22% of patients had a final diagnosis of protein-losing enteropathy, 43% had a diagnosis of eosinophilic esophagitis, and 21% had eosinophilic enteritis. The response to elimination diet therapy was high (clinically in 82%, histological improvements were noted in 78% of cases), which implies an allergic etiology of the disease. This study highlights the importance of morphological evaluation of the biopsy for diagnosis of the disease, since unlike EoE, eosinophilic infiltration is rarely limited to one organ (stomach only or small intestine only) - most often the stomach and small intestine are simultaneously affected (eosinophilic gastroenteritis). The clinical picture depends on which layer of the digestive tube is infiltrated by eosinophils. If only the mucous membrane is involved in the pathological process, symptoms such as abdominal pain, nausea, vomiting and diarrhea dominate. If eosinophils are concentrated in the muscle layer, symptoms of obstruction (nausea, vomiting, bloating) come to the fore. If eosinophils are concentrated in the submucosal layer, the disease manifests itself as ascites, and a large number of eosinophils are found in the ascitic fluid. Examination of biopsy tissue is informative only in cases of damage to the mucous membrane. Macroscopically, erythema, erosion, nodularity, or polypoid growths are detected in the antrum of the stomach and/or small intestine. On histological examination, the inflammatory infiltrate is dominated by eosinophils, the number of which exceeds 20 cells per field of view (×400). Since pathological changes are focal in nature, it is necessary to take at least 5 biopsies from each section of the digestive tube, including both altered and apparently normal areas of tissue.
Eosinophilic gastroenteritis
Eosinophilic gastroenteritis is accompanied by malabsorption and protein loss, as well as iron deficiency anemia due to intestinal bleeding. Penetration of eosinophils into the submucosal and muscular layers can cause a complication such as eosinophilic ascites. In 25–50% of cases of the disease, food allergy occurs [23].
Eosinophilic gastroenteritis is a rare inflammatory disease. In addition to anemia, clinical manifestations include dyspepsia and diarrhea. In a gastric biopsy, tissue infiltration with eosinophils is detected.
Identification of food allergens, an elimination diet and corticosteroid therapy (mainly in the form of metered-dose inhalers used for bronchial asthma) are considered an effective approach in the treatment of eosinophilic gastrointestinal diseases [6, 7].
In the Russian literature, only a few publications are devoted to this nosology [5]. This problem is especially acute for pediatrics. We provide a brief description of a clinical case of an undiagnosed eosinophilic gastrointestinal disease in a child aged 1 year 2 months (the child was examined in December 2015 in one of the children's clinical hospitals in Moscow).
A child from the fourth pregnancy, which was threatened with miscarriage in the first trimester, acute respiratory viral infection at 5 months, preeclampsia at the end of pregnancy. The second birth is urgent, with stimulation. Birth weight - 3810 g, height - 53 cm, Apgar 8/9 points. From birth to 6 months the child was breastfed, then bottle-fed. Complementary feeding from 6 months. Early development without features. Among the diseases suffered: left-sided pyeloectasia, acute respiratory viral infection 3 times, phimosis, umbilical hernia, planovalgus foot deformity. Vaccinated according to age, allergy history is not burdened. Parents consider themselves healthy.
Up to 6 months weight gain up to 1 kg. He ate willingly, often vomited profusely, “vomiting like a fountain.” Ultrasound at 1 month revealed kinking of the gallbladder, left-sided pyelectasia (pelvis 3.5 mm). I received Nutrilon Antireflux (dietary supplement) at every feeding, Lactase Baby (dietary supplement), Phosphalugel, Creon - without effect. From birth, stool with a tendency to constipation, 1 time every 2-3 days more often after microlax, undigested with mucus, anxiety during feeding, intestinal colic. In the coprogram there is minor steatorrhea of the 1st type. At 3 months I received Motilium and Magnesium Donat - no effect. While taking Creon, there is a decrease in flatulence and the stool is more digested. Complementary feeding from 6 months - dairy-free oatmeal, buckwheat, rice, vegetables: broccoli, cauliflower, zucchini; fruits: apple, pear - no reaction. From 7 months cottage cheese, chicken meat. Cow's milk - from 8 months - flatulence, kefir - less flatulence. With the introduction of complementary foods, the frequency of vomiting decreased. Currently, she burps rarely, once a month. Stool 1 time per day, undigested, formed. On admission: condition was satisfactory. weight - 13 kg, height 81 cm. Objectively, the child has bone changes: pronounced deformation of the legs, late teething (at 1 year - 2 teeth). Internal organs - without pronounced deviations. A biochemical blood test on October 5, 2015 revealed hypoproteinemia up to 57.5 g/l (56–80), hyperphosphatemia up to 1.70 μmol/l (normal 0.65–1.32). Immunoglobulins in blood serum: IgG 3.5 (3.5–10.0), IgE 27.6 (0–60.0), IgM 0.6 (0.7–1.6), IgA 0.4 ( 0.4–1.4). Allergen-specific IgE: sensitization to egg white, yolk, beta-lactoalbumin, alpha-lactoalbumin, casein, cow's milk, wheat flour, oatmeal was not detected. Blood test for eosinophilic cationic protein - 30.6 ng/ml (normal range 0–24).
FEGDS: the fiberscope is inserted freely. The mucous membrane of the esophagus is pale pink, smooth. The cardia closes. Line 2 is within normal limits. The stomach contains a small amount of liquid contents, light in color. The folds are located longitudinally and are straightened with air. The mucous membrane is pink, smooth. Peristalsis is observed. The pylorus is round in shape when opened. The mucous membrane of the duodenal bulb is pink and loose. The mucous membrane of the postbulbar sections of the duodenum is pink, loose, with lymphangiectasias. The folds are normal. BDS has not been changed. Conclusion: moderately severe duodenitis.
Results of a morphological study of the upper gastrointestinal tract. Serial sections of the duodenal mucosa without the muscular plate. The cuts are tangential, with up to three villi in the cut. The villi have scalloped edges and the goblet cells are unevenly distributed. The density of the cellular infiltrate of the lamina propria is not increased; there is a significant number of eosinophils. Focal dilatation of lymphatic vessels in the lamina propria. Paneth cells are present in sufficient numbers. Conclusion: no signs of inflammatory changes or celiac disease were found in the presented preparations.
Ultrasound of the abdominal organs. Liver: enlarged, right lobe 90.5 mm, left 60 mm, smooth, clear contours, homogeneous structure, sharp edges, increased echogenicity. The vessels are full of blood, the bile ducts are not changed. The gallbladder is of normal shape, the walls are normal, and there is homogeneous bile in the cavity. Pancreas: not enlarged, dimensions: head 10.3 mm × body 7 mm × tail 10.4 mm, clear contours, increased echogenicity, homogeneous structure. Wirsung's duct is 1.5 mm.
The child was discharged with a final diagnosis: vitamin D3 deficiency rickets, residual effects. Food sensitization, intestinal form, not IgE-mediated. Duodenitis. Reactive pancreatitis, interstitial form. Selective deficiency of IgA and IgM. Muscular dystonia syndrome.
Literature
- Burks A., Tang M., Sicherer S. et al. ICON: Food allergy // J Allergy Clin Immunol. 2012; 129:906–920.
- Sampson H., Aceves S., Bock A. et al. Food allergy: A practice parameter update-2014 // Ibid. 2014; 134:1016–1025.
- Turnbull J., Adams H., Gorard D. The Diagnosis and Management of Food Allergy and Food Intolerances // Aliment Pharmacol Ther. 2015; 41:3–25.
- Bird J., Lack G., Perry T. Clinical Management of Food Allergy // J Allergy Clin Immunol Pract. 2015; 3:1–11.
- Sopo M., Iacono D., Monicaa G., Giovannac M. Clinical management of food protein-induced enterocolitis syndrome // Curr Opin Allergy Clin Immunol. 2014; 14: 240–245.
- Liacouras C., Furuta G., Hirano I. et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults // J Allergy Clin Immunol. 2011; 128:3–20.
- Dellon E., Gonsalves N., Hirano I. et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) // Am J Gastroenterol. 2013; 108:679–692.
- Spergel J. Patients With Previous Food Allergies at Risk for Esophagitis // Medscape. 2014, Mar 11.
- Ram G., Lee J., Ott M. et al. Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis // Ann Allergy Asthma Immunol. 2015; 115:224–228.
- Ivashkin V. T., Baranskaya E. K., Kaibysheva V. O., Ivanova E. V., Fedorov E. D. Eosinophilic esophagitis: review of the literature and description of our own observation // RZHGGK. 2012. T. 22. No. 1. P. 71–81.
- Sadikov I. S., Macharadze D. Sh., Khomeriki S. G. Features of the diagnosis of eosinophilic esophagitis // Experimental and clinical gastroenterology. 2015, 114, p. 52–59.
- Miehlke S. Clinical features of Eosinophilic esophagitis in children and adults // Best Pract Res Clin Gastroenterol. 2015; 29: 739–748.
- Straumann A., Aceves S., Blanchard C. et al. Pediatric and adult eosinophilic esophagitis: similarities and differences // Allergy. 2012; 67:477–490.
- Fashner J., Gitu A. Common gastrointestinal symptoms: dysphagia // FP Essent. 2013; 413: 11–15.
- Dellon E. Diagnostics of eosinophilic esophagitis: clinical, endoscopic, and histologic pitfalls // Dig Dis. 2014; 32:48–53.
- Hirano I., Moy N., Heckman MG et al. Endoscopic assessment of the oesophageal features of eosinophilic esophagitis: validation of a novel classification and grading system // Gut. 2013; 62:489–495.
- Dellon E., Kim H., Sperry S. et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease // Gastrointest Endosc. 2014; 79:577–785.
- Aceves S., Newbury R., Dohil R. et al. Esophageal remodeling in pediatric eosinophilic esophagitis // J Allergy Clin Immunol. 2007; 119:206–212.
- Gonsalves N., Policarpio-Nicolas M., Zhang Q. et al. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis // Gastrointest Endosc. 2006; 64:313–319.
- Salek J., Clayton F., Vinson L. et al. Endoscopic Appearance and Location Dictate Diagnostic Yield of Biopsies in Eosinophilic Oesophagitis // Aliment Pharmacol Ther. 2015; 41:1288–1295.
- Moawad F., Schoepfer A., Safroneeva E. et al. Eosinophilic Oesophagitis and Proton Pump Inhibitor-Responsive Oesophageal Eosinophilia Have Similar Clinical, Endoscopic and Histological Findings // Ibid. 2014; 39:603–608.
- Ko H., Morotti R., Yershov O. et al. Eosinophilic Gastritis in Children. Clinicopathological Correlation, Disease Course, and Response to Therapy // Am J Gastroenterol. 2014; 109:1277–1285.
- Ekunno N., Munsayac K., Pelletier A., Wilkins T. Eosinophilic Gastroenteritis Presenting with Severe Anemia and Near Syncope // Am Board Fam Med. 2012; 25:913–918.
D. Sh. Macharadze, Doctor of Medical Sciences, Professor
Federal State Budgetary Educational Institution of Higher Professional Education RUDN University, Moscow
Contact Information
How to take the test correctly?
In order for the analysis result to be accurate and truly reflect what is happening in our body, we need to be properly prepared. Moreover, there is nothing complicated in preparing for this analysis.
First of all, both parents and the child need to prepare mentally. It is best for the child not to cry, not to panic, and to behave calmly. To do this, parents should explain to the baby what will happen in the hospital, that there is nothing wrong with it. Maybe you can even promise your child something in return if he behaves well.
It is also important to prevent your child from running around the hospital corridors while waiting for their turn in the blood collection room. Physical activity may affect study results.
Also, one of the most important rules for preparing for a blood test is that it must be taken on an empty stomach. If the child is already big (over 4 years old), then you can be patient and donate blood after an overnight fast. It is allowed to give the child water to drink.
It is recommended not to feed infants for 1 - 1.5 days before donating blood.
Blood is most often taken from the finger; in very small children, from the heel.
When preparing to donate blood, it is important to take prescribed medications. A number of medications can affect test results. Therefore, it is advisable to talk to your doctor about this. Don't do anything on your own!
Some medications can affect the level of the determined indicator. For example, Prednisolone can lead to a decrease in the level of eosinophils and monocytes in the blood.
If parents properly prepare for donating blood, they will not have to take the test again, plunging their child into a stressful situation.
Which blood test shows the level of eosinophils?
The exact number of eosinophils is indicated in the results of a blood test with a leukocyte formula. Blood is taken from a vein or finger for analysis. The study is carried out under a microscope using various reagents and dyes. During this analysis, the number of all types of leukocytes per unit volume of blood is calculated, as well as the percentage of each type of leukocyte. A leukogram (leukocyte formula) allows you to see the increase and decrease in the number of one or another type of leukocyte.
Interpretation of results
The interpretation of the results should be carried out by the attending physician who referred your child for a blood test. If the parents independently asked for a blood test, then deciphering the answer should be entrusted to a specialist. It may be located in the same place where the blood was donated, or you can contact your place of residence with a ready-made test result.
When eosinophils are elevated in a child and in an adult, this condition is called eosinophilia. Next we will look at situations where this is possible and why it occurs.
Why are eosinophils in a child’s blood elevated?
There are a number of conditions when eosinophils are elevated in the blood.
- A worm entered the body, that is, helminthiasis occurred. Eosinophils migrate to the lesion, trying to get rid of the unwanted “guest”, thereby increasing their number in the blood. The most common parasites in children are pinworms and roundworms.
- Allergic reactions. In response to the penetration of an allergen into the child’s body, an immune reaction develops, during which various cells are activated. These include eosinophils. As mentioned earlier, they help break down histamine, an allergy substance. In order to confirm the presence of an allergic reaction, the level of eosinophils in the blood is determined. If the allergy is confirmed, they move on to searching for what could have caused it.
- Allergic diseases. This group includes pathologies such as bronchial asthma, hay fever and others. They are already “fixed” in the body, they are more difficult to get rid of than simple allergies.
- Hypersensitivity to drugs. It usually occurs immediately after administration of the drug. Such people then have to mention this throughout their lives when contacting a medical organization.
- Lefler's syndrome. This pathology is associated with the formation of an infiltrate in the lungs, which can be seen during a chest x-ray. In parallel, there is a high level of eosinophils in the blood. However, this pathology is very rare, most often in older people.
- Hypereosinophilic syndrome. This condition is accompanied by an excess amount of eosinophils in the blood and damage to the corresponding tissues. No parasites or allergic diseases are detected in the patient. What causes this condition remains unknown.
- Pathology of connective tissue. This includes: vasculitis, systemic lupus erythematosus, scleroderma and others. An increased level of eosinophils indicates the presence of a developing inflammatory process.
- Malignant neoplasms , for example, leukemia, can lead to eosinophilia.
- Polycythemia is accompanied by an increase in all blood cells, eosinophils are no exception. Diagnosing this disease is not difficult.
- Acute bacterial infections , infectious mononucleosis, tuberculosis can cause an increase in the level of eosinophils in the blood.
Major eosinophilia
The group of large lesions includes diseases in which the level of eosinophils is increased and amounts to more than 15–20%. Monocytosis and general leukocytosis develop simultaneously.
Monocytes also belong to granulocytes; their norm is higher than eosinophils (up to 13%). A simultaneous increase in monocytes and eosinophils occurs when encountering a severe infection, providing a pronounced response to the introduction of helminths.
Infectious eosinophilosis is a disease with an unknown cause. It has a wavy course, acute or subacute onset. Severe fever, runny nose, joint pain.
Tropical eosinophilia develops in hot climates, a high temperature lasts for a long time, a dry cough, and asthmatic breathing appear. Up to 80% of eosinophils are found in the blood. The disease is classified as a parasitic infection.
Actions of parents in case of eosinophilia in children
If elevated levels of eosinophils are detected, parents should consult a doctor. Since this is a “bell” that something is going wrong in the child’s body.
If it is confirmed that the child has a parasite, the doctor will prescribe medications to help remove it from the body. Do not drug your child without consulting a doctor!
If an allergic reaction is confirmed, it is important to identify its source. Then remove the child from contact with this allergen.
In general, in any case, consult a doctor, independence can aggravate the situation.
Why do eosinophils increase?
In children, the most common cause of increased eosinophils is infection with worms and allergic reactions. Worm infestation is a common disease due to the child’s failure to comply with hygienic rules of hand washing and frequent contact with infected animals. Allergies can develop to food, milk, fruits.
It won’t take long for babies to learn hygiene
Other reasons:
- infection with a fungal infection, staphylococcus;
- lack of magnesium in the body;
- skin diseases (lichen, dermatitis, psoriasis);
- blood diseases;
- vascular damage (vasculitis);
- malignant tumors.
Separately, congenital, genetically determined eosinophilia is distinguished.