Pulmonary atresia: causes
Pulmonary atresia is one of the most complex and dangerous heart diseases. The frequency of this pathology is approximately 7 cases per 100 thousand newborns. Among all congenital heart pathologies, the disease accounts for 1-3%.
The formation of the defect occurs when there is an abnormal fusion of the endocardial ridges, which are the rudiments of the semilunar valves. In the future, this can cause fusion of the pulmonary artery.
The etiology of the disease is still unclear. According to one assumption, unfavorable factors affecting a woman’s body during pregnancy play a certain role, as a result of which embryogenesis is disrupted. For example, a pregnant woman's use of hormonal medications and antibiotics, smoking, or drinking alcohol and drugs can have a negative impact on the development of the fetal cardiac system.
During the antenatal period, the release of blood into the trunk of the pulmonary artery from the right ventricle is sharply disrupted, which is caused by the fusion of the pulmonary valve flaps. As a result, the pressure in the right ventricle increases, the filling of the embryonic sinusoidal-coronary messages of the right ventricle increases. Blood can escape from there only in case of triscupid insufficiency. This insufficiency is the cause of a significant increase in the size of the right atrium.
Pulmonary atresia in children: statistics, forecasts
In children with pulmonary atresia, there is no valve that allows blood to flow directly from the right ventricle into the pulmonary artery, or the valve does not open, so almost all the blood from the right atrium is shunted through the patent foramen ovale into the left atrium. Part of the blood, in the presence of pronounced sinusoids from the right ventricle, enters the coronary bed retrogradely. This leads to a decrease in oxygen content in the coronary blood and increases the risk of developing myocardial hypoxia.
The child's survival is only possible if there is an alternative blood supply to the lungs, when the left ventricle pumps blood into both circulations. The general prognosis of the disease is extremely unfavorable: approximately half of babies with pulmonary atresia die in the first two weeks, the rest die in the first year of life.
Conservative treatment of pulmonary atresia is not effective. Only an operation that eliminates obstruction at the level of the pulmonary artery trunk and restores the efficiency of pulmonary blood flow can save a newborn from a pulmonary artery fusion.
Publications in the media
Tricuspid valve atresia (ATA) is a congenital heart disease with a lack of communication between the right atrium and ventricle. Statistics: 1.6–3% of all congenital heart disease diagnosed in infancy.
Etiology • Displacement of the interventricular septum relative to the atrioventricular canal. As a result, in the absence of development of the sinus part of the right ventricle, the interventricular septum, moving to the right, leads to obliteration of the right atrioventricular orifice • See also Tetralogy of Fallot.
Pathogenesis • The defect is always accompanied by interatrial communication (patent foramen ovale or true atrial septal defect [ASD]), hypoplasia or aplasia of the right ventricle, as well as communication between the systemic and pulmonary circulations at the level of the ventricles (VSD or single ventricle of the heart) or great vessels ( patent ductus arteriosus [PDA], aortopulmonary septal defect) • Hemodynamic disturbances depend on the anatomical variant (see below) and do not depend on the form of ATC (see below) • With ATC with reduced pulmonary blood flow, patients have pronounced hypoxemia, and pulmonary blood flow is practically does not depend on the resistance of the pulmonary vessels and is completely determined by the total area of the opening of the pulmonary valve and the PDA • In ATC with normal or increased pulmonary blood flow, the latter depends on the dynamic resistance of the pulmonary vessels, compensatory decreasing as the natural arteriovenous communications narrow (obliteration of the PDA and, less commonly, VSD ) • Because, at a constant cardiac output, a rapid increase in pulmonary blood flow leads to a proportional decrease in systemic blood flow, this can lead to systemic hypoperfusion and acidosis • A more gradual increase in pulmonary blood flow can lead to respiratory distress and damage to the pulmonary microvasculature.
Anatomical variants of ATC • ATC with normal or increased pulmonary blood flow •• Normal location of the great vessels, VSD and absence of pulmonary artery stenosis •• Atresia of the pulmonary trunk and PDA •• VSD, transposition of the great vessels without pulmonary stenosis •• Transposition of the great vessels, ostial atresia aorta and PDA •• Transposition of the great vessels, VSD, absence of pulmonary stenosis, bicuspid or tricuspid mitral valve • ATK with reduced pulmonary blood flow •• Normal location of the great vessels, VSD and pulmonary stenosis •• Normal location of the great vessels, pulmonary atresia and PDA •• Transposition of the great vessels, VSD and pulmonary stenosis •• Transposition of the great vessels, VSD and right ventricular hypoplasia •• Transposition of the great vessels, VSD, pulmonary stenosis, right ventricular hypoplasia, tricuspid mitral valve.
Forms of ATC • Muscular form (76–100%): the blindly ending bottom of the right atrium is located above the free wall of the left ventricle (atrioventricular discordance), the right ventricle is hypoplastic due to the absence of its sinus section • Membrane form (7–12%): bottom of the right atrium is located above the atrioventricular part of the interventricular septum, the right ventricle is hypoplastic due to the absence of its sinus section • Valve form (4.8–6%): between the right atrium and the ventricle there is a fibrous membrane, represented by completely fused leaflets of the tricuspid valve (atrial ventricular concordance), the right ventricle is hypoplastic, but fully formed, a rudimentary valve apparatus can sometimes be found in it • Atresia of the Ebstein anomaly type (2–8%): the tricuspid valve, displaced into the cavity of the right ventricle, has cusps fused and spread along the wall of the right ventricle • Atresia of the patent AV canal type (isolated cases): the leaflets of the common atrioventricular valve block the exit from the right atrium.
Clinical picture. The presence or absence of hypoxemia is determined by the magnitude of pulmonary blood flow, and circulatory failure is determined by the magnitude of intersystem shunts. Therefore, the symptoms described below are variable and may occur in different combinations in different patients • Complaints •• Retarded physical development •• Constant shortness of breath or dyspnea-cyanotic attacks • Objectively •• Shortness of breath at rest, aggravated by physical activity •• Retarded physical development • • Systemic cyanosis •• Symptoms of “drum sticks” and “watch glasses” •• Signs of circulatory failure in the systemic circle (hepatomegaly, edema, etc.) •• Expansion of all boundaries of the heart •• Systolic murmur in the third and/or fourth intercostal spaces on the left from the sternum, caused by VSD •• Systolic murmur of pulmonary artery stenosis in the second intercostal space to the right or left (with transposition of the great vessels) from the sternum •• Diastolic murmur of relative stenosis of the mitral valve (Coombs’ sign) at the apex of the heart •• In adult patients, murmurs may not listen.
Instrumental diagnostics • ECG •• There are no specific symptoms of ATC •• ECG changes are caused by concomitant anomalies (see Atrial septal defect, Ventricular septal defect, Patent ductus arteriosus, Transposition of the great vessels, Pulmonary valve stenosis) • Chest X-ray •• Severity pulmonary pattern depends on the anatomical variant of the defect •• With hypo- and aplasia of the right ventricle - retraction of the arch of the right ventricle •• Bulging of the arches of the heart •• See also Transposition of the great vessels • EchoCG •• Inability to visualize the tricuspid valve leaflets and transtricuspid flow •• Decreased size or absence of the right ventricle •• Define some criteria for the success of hemodynamic correction of the defect (see below) •• See also Atrial septal defect, Ventricular septal defect, Patent ductus arteriosus, Transposition of the great vessels, Pulmonary valve stenosis • Cardiac catheterization •• Catheter is not conducted from the right atrium to the right ventricle, but easily passes into the left atrium and further into the left ventricle •• Increased pressure in the right atrium •• Decreased blood oxygen saturation in the left atrium •• See also Atrial septal defect, Ventricular septal defect, Patent ductus arteriosus, Transposition of the great vessels, Pulmonary valve stenosis • Right and left atriography and ventriculography, ascending aortography •• Light triangle sign (clearance at the site of the absence of the inflow tract of the right ventricle) •• See also Atrial septal defect, Ventricular septal defect, Patent ductus arteriosus, Transposition of the great vessels, Pulmonary valve stenosis.
Drug therapy •• Treatment of dyspnea-cyanotic attacks - see Tetralogy of Fallot •• Treatment of circulatory failure •• See also Transposition of the great vessels.
Surgery
• Indications: all patients with ATC.
• Contraindications •• Irreversible pulmonary hypertension (with associated anomalies) •• Severe concomitant pathology that threatens the patient’s life •• Terminal stage of circulatory failure •• Contraindication to radical correction - aplasia of the right ventricle, relative contraindication - impossibility of correction of concomitant defects •• Also indications for hemodynamic correction for a particular patient is determined using the Schuss operability criteria.
• Operability criteria for hemodynamic correction of ATC •• Minimum age of the patient - 4 years •• Sinus rhythm •• Vena cava drainage is normal (into the right atrium) •• The volume of the right atrium is not increased •• The average pressure in the pulmonary trunk is 15 mm Hg •• Total pulmonary vascular resistance is not more than 4 units/m2 •• The ratio of the diameters of the pulmonary trunk and aorta is not less than 0.75 •• The ejection fraction of the left ventricle is not less than 60% •• The function of the mitral valve is not impaired •• Previously, intervascular anastomoses were not created.
• Methods of surgical treatment •• Palliative interventions - see Transposition of the great vessels •• With increased pulmonary blood flow, accompanied by respiratory distress syndrome or general acidosis, an operation is performed to narrow the pulmonary trunk using a cuff •• With reduced pulmonary blood flow, systemic-pulmonary anastomoses are applied ( see Tetralogy of Fallot), and for patients over 10 years of age, a bidirectional cavapulmonary anastomosis is created or a Hemi-Fontan operation is performed •• For hypoplasia of both pulmonary arteries, reconstruction of the outflow tract from the right ventricle is performed with a patch of autopericardium or synthetic material •• Radical surgical treatment - hemodynamic correction ( Fontan operation): ••• repair of atrial septal defect; ••• right atriopulmonary shunting (with concomitant transposition of the great vessels, hypoplasia or atresia of the pulmonary trunk, severe hypoplasia of the right ventricle) or AV shunting; ••• implantation of a homovalve at the mouth of the inferior vena cava; ••• creation of a complete cavapulmonary anastomosis with exclusion of the right chambers of the heart from the circulation.
Specific postoperative complications: ascites, hydrothorax, acute renal failure.
Prognosis • By the first year of life, 75–90% of patients die • Hospital mortality after hemodynamic correction is 5–20%, depending on the operability criteria (see above): if all ten criteria are met, the average mortality rate is 4.2%, and if not met all ten - 18.9% • When the average atrial pressure decreases in the next 2 weeks after surgery below 15 mm Hg. the risk of developing circulatory failure in the systemic circle increases due to a decrease in cardiac output (ascites, hydrothorax, hepatomegaly) • AKI in the early postoperative period develops in 26–31% of patients • Physical performance in the early stages after the Fontan operation corresponds to 30–42% of the age norm, after 1 year - 70% • Functional parameters of the heart, as a rule, remain reduced in all patients, and their changes in response to stress are not always adequate, but this does not significantly affect their quality of life • In the long term, up to 6.8– 12.5% of patients, mainly from chronic heart failure and liver cirrhosis. Half of these patients have to undergo repeated interventions for stenoses and occlusions of valveless or valve-containing prostheses • There are isolated cases of life expectancy of those operated on up to 30 and 60 years.
Abbreviations. ATK - tricuspid valve atresia. PDA - patent ductus arteriosus.
ICD-10 • Q22 Congenital anomalies [malformations] of the pulmonary and tricuspid valves
Symptoms of pulmonary atresia
Symptoms of the disease usually appear within the first few hours of a child's life. Sometimes the disease appears a few days after birth. Its main features are:
- increasing bluish tint of the skin (cyanosis);
- difficulty breathing;
- dyspnea;
- fatigue when feeding.
The life expectancy of babies born with pulmonary atresia largely depends on the diameter of the patent ductus arteriosus, through which blood enters the pulmonary artery.
Treatment
The choice of treatment for pulmonary atresia is directly related to the specific characteristics of the right ventricle and pulmonary artery. The most effective is surgical treatment.
- Drug treatment (does not eliminate the problem, usually prescribed before surgical treatment).
- Surgical treatment (often carried out in several stages, each of which is carried out at certain intervals):
- endovascular operations (a balloon catheter is inserted through the femoral artery to the site of narrowing of the channel through which blood leaves the right ventricle; its inflation leads to improved hemodynamics);
- open heart surgery (during the operation, a connection is established between the right ventricle and the pulmonary artery).
Diagnosis of pulmonary atresia
Making a diagnosis begins with a medical examination. Using a stethoscope, the doctor carefully listens to the heart. In case of any abnormalities in cardiac activity, murmurs can be heard. A detailed survey about the symptoms and characteristics of the course of the disease, and a study of family history of diseases help the doctor make a preliminary diagnosis.
For further diagnosis, the following types of studies are used:
- electrocardiography - allows you to identify signs of heart overload, including its right parts, and determine the presence of right ventricular hypertrophy;
- chest x-ray - helps determine the increase in the size of the heart tissue, as well as characteristic changes in the pulmonary pattern;
- echocardiogram - the study can determine the degree of disturbance in the structure of the right ventricle, as well as identify insufficiency of the tricuspid valve and open arterial flow. Color mapping provides visualization of pathological blood flow, determines its speed and volume;
- phonocardiography - records pathological noises caused by hemodynamic disturbances. In this way, the murmur of tricuspid regurgitation can be detected;
- catheterization of the cardiac cavities - this invasive study is aimed at performing direct manometry, which makes it possible to determine the pressure in both ventricles, as well as other parts of the heart;
- angiocardiography is an x-ray study that allows selective examination of parts of the heart and large vessels. During the procedure, a contrast agent is injected into the bloodstream in the area under study, filling the lumens of blood vessels and the cavities of the heart, which makes it possible to obtain an image with clear contours.
Catalog
Cardiac surgeon, scientist, teacher and organizer of science, Doctor of Medical Sciences (1973), Professor (1982), Academician of the Russian Academy of Medical Sciences (1994), Academician of the Russian Academy of Sciences (2011), Honored Scientist of the Russian Federation (1994) , laureate of the Lenin Prize (1976), State Prize of the USSR (1986), State Prize of the Russian Federation (2002), Prize of the Government of the Russian Federation (2003).
In 1994, L.A. Bockeria was elected through a competition to the position of director of the National Research Center for Agricultural Sciences named after. A.N. Bakuleva. In 1998, he simultaneously became director of the Center for Surgical and Interventional Arrhythmology of the Ministry of Health of the Russian Federation.
Since 1994, he has been the head of the Department of Cardiovascular Surgery at the Russian Medical Academy of Postgraduate Education (RMAPO) of the Ministry of Health of the Russian Federation. Since 1995, he has been the head of the Department of Cardiovascular Surgery No. 2 of the Moscow Medical Academy (now the First Moscow State Medical University), which he created. THEM. Sechenov Ministry of Health of the Russian Federation.
Since 2003, he has headed the Department of Cardiovascular Surgery and Interventional Cardiology at the Moscow State Medical and Dental University. A.I. Evdokimov Ministry of Health of the Russian Federation.
On the initiative of L.A. Bokeria at the National Medical Research Center for Agricultural Sciences successfully operates an educational and research center that annually conducts 4–6 courses of postgraduate advanced training for senior specialists.
L.A. Bokeria performs the entire known arsenal of heart surgeries for a wide variety of pathologies: from 3 to 6 operations per day, that is, from 700 to 900 operations using artificial circulation per year.
Since 1996, he has been the chief cardiac surgeon of the Ministry of Health of the Russian Federation.
L.A. Bockeria is the author and co-author of over 3,700 scientific publications, including more than 250 books, more than 100 inventions and utility models, more than 300 computer programs and databases, a number of which are registered abroad, on various problems of cardiovascular surgery, cardiology, medicine education and organization of medical science.
L.A. Bockeria owns unique works on the theoretical substantiation and clinical use of the hyperbaric oxygenation method in heart and vascular surgery. He is a leading specialist in the field of diagnosis and surgical treatment of cardiac rhythm and conduction disorders (especially tachyarrhythmias), including various combinations of cardiac arrhythmias with congenital and acquired heart defects and anomalies, coronary disease.
L.A. Bokeria is the initiator of the development of another new section of cardiac surgery in our country - minimally invasive heart surgery. L.A. made a great contribution. Bockeria in solving the problem of surgical treatment of coronary artery disease.
The role of L.A. is great. Bockeria in the development of new approaches to surgical treatment of terminal heart failure. His merit is the development of new operations in a severe category of cardiac surgical patients with various forms of cardiomyopathies. He was the first to develop the concept of dynamic cardiomyoplasty, including in children. L.A. Bockeria performed the first implantation of an artificial heart ventricle in our country and, after a long break, initiated the resumption of heart transplant operations at the Center.
On the initiative of L.A. Boqueria introduced advanced technologies for diagnosis and reconstructive surgery of diseases of the ascending aorta and arch into clinical practice.
He generated the development of another direction of modern medical science - creative cardiology, which determines the need for creative cooperation between doctors of different specialties: cardiologists, cardiac surgeons, specialists in functional diagnostics, fundamental and applied disciplines.
Under the leadership of L.A. At the Boqueria Center, new high-tech principles for the prevention and treatment of patients with cardiovascular pathology are being developed and introduced into clinical practice - the use of gene and cell therapy methods.
Academician L.A. Boqueria is conducting priority research on the creation of bioprosthetic heart valves for the correction of valve defects - a low-profile, curved bioprosthesis of the mitral and tricuspid valves, reproducing the natural shape of the fibrous ring, on an elastic frame. The study of the effectiveness of myocardial protection in children of the first year of life with the use of the new intracellular cardioplegic solution “Bokeria-Boldyreva”, created at the National Medical Research Center for Cardiovascular Surgery under the leadership of L.A., continued. Boqueria.
L.A. Boqueria is actively involved in the methodology of medical science and teaching activities. He is the founder of the country's largest cardiac surgery school, having trained more than one generation of doctors - cardiac surgeons, cardiologists, resuscitators and specialists in other related specialties.
L.A. Bockeria is the scientific supervisor of 350 candidate dissertations and a consultant of more than 100 doctoral dissertations. He is the founder of the publishing house NMITSSSH named after. A.N. Bakuleva with the printing house, founder and editor-in-chief of the journals “Annals of Surgery”, “Bulletin of the National Medical Center for Cardiovascular Surgery named after. A.N. Bakulev “Cardiovascular diseases”, “Childhood diseases of the heart and blood vessels”, “Clinical physiology of blood circulation”, “Annals of Ari, information collection “Cardiovascular surgery”; Editor-in-chief of the journal "Thoracic and Cardiovascular Surgery".
Active work of L.A. Boqueria and his contribution to domestic healthcare have been awarded titles and awards of the highest value. He is a laureate of the Lenin Prize (1976), two State Prizes (1986 - USSR, 2002 - Russian Federation), and the Prize of the Government of the Russian Federation (2003). For outstanding achievements of L.A. Bockeria was awarded the Order of Merit for the Fatherland, III (1999), II (2004) and IV (2010) degrees, the Order of Dignity and Honor (Republic of Georgia, 1999), the Order of St. Sergius of Radonezh, II degree (2001). The Russian Biographical Institute has repeatedly recognized L.A. Bokeria “Person of the Year”, and in 2000 - “Person of the Decade” in the “Medicine” category. In 2002, he was awarded the title “Legend Man”, the all-Russian “Russian National Olympus” award, established by the Government, the Union of Industrialists and the Third Millennium Foundation. As one of the leading cardiac surgeons in the world in 2003, L.A. Bokeria was awarded the international Golden Hippocrates award. In 2004, he was awarded the RAS Triumph Prize in the Life Science – Medicine category. In 2004, he was awarded the Order of Patron, which is awarded by the Patrons of the Century Charitable Foundation for his outstanding contribution to the revival and prosperity of the world, for the greatness of the soul, for selfless generosity; in 2004 and 2005 – the Golden Badge of Honor “Public Recognition”, which is awarded by the National Foundation for “Public Recognition”, the National Civil Committee for Interaction with Law Enforcement, Legislative and Judicial Bodies and the independent organization “Civil Society” for great personal contribution to the development of the domestic medicine, conducting unique cardiac surgery using the latest medical technologies that saved the lives of hundreds of children and newborns, many years of fruitful scientific, practical, pedagogical and educational activities, active citizenship.
In 2006, L.A. Boqueria was awarded the Honorary Diamond Order “Public Recognition”, awarded a diploma from the Presidium of the Parliament of the Peoples of Russia “for saving numerous lives, outstanding knowledge, ability to lead, for unique personal qualities - nobility, courage, sense of duty, ability to preserve honor and dignity, keep one’s word and do business, also for faith in the great future of the Fatherland,” a Gold Medal for outstanding contribution to the education of Russia and a Gold Star “Honor, Pride and Glory of Russia.”
In 2008, L.A. Bockeria was awarded the medal “For practical contribution to strengthening the health of the nation”, the Order of Honor with the title “Support of Honest Business” and the honorary title “Outstanding Cardiac Surgeon of Our Time”. In 2009, the huge contribution of L.A. Bockeria's contribution to science and domestic healthcare was awarded the A.N. Prize. Kosygin “For great achievements in solving problems of the development of the Russian economy” and the Moscow City Prize in the field of medicine “for the development and introduction into clinical practice of a new biological valve “Bioglis”.
L.A. Bokeria is a full member of the American Association of Thoracic Surgeons (1991), member of the board (1992) and member of the Presidium (since 2003, consul) of the European Society of Thoracic and Cardiovascular Surgeons, member of the board of the European Society of Cardiovascular Surgeons, member of the scientific board of the International Cardiothoracic Center of Monaco (1992), member of the Serbian Academy of Sciences (1997), honorary member of the American College of Surgeons (1998), academician of the Academy of Medical Sciences of Ukraine, honorary professor of Moscow State University. M.V. Lomonosov (2011), foreign member of the National Academy of Sciences of Georgia (2012).
He is the president of the Association of Cardiovascular Surgeons of Russia (1995), president of the All-Russian public organization “League of National Health” (2003), member of the Public Chamber of the Russian Federation of all convocations.
Treatment of pulmonary atresia
If the diagnosis of pulmonary atresia or pulmonary artery fusion is confirmed, treatment should begin immediately. Proper treatment started in a timely manner increases the baby’s chances of survival.
Treatment of pulmonary atresia with medications is not a radical method and is used only as maintenance therapy before surgery. This treatment is aimed at reducing the body's oxygen consumption and correcting metabolic disorders.
The choice of surgical intervention depends on the degree of change in the anatomical structure of the pulmonary artery and right ventricle. In case of minor deformation of the heart, a transthoracic approach is used in the area of the fourth intercostal space on the left. The operation is performed under general anesthesia and lasts several hours. Having gained access to the heart, the surgeon expands the right ventricular outlet using a special installation - a transanular patch.
After surgery, the symptoms of the disease completely disappear, as the release of blood into the pulmonary artery is normalized. During the operation, the surgeon’s actions may be aimed at blocking the open arterial flow due to the fact that after correction of atresia it becomes irrelevant. Closing the flow can be achieved not only surgically, but also through the administration of special medications. An improvement in the patient's condition is observed already in the first hours after the operation.
If the right ventricle is severely deformed, it is not possible to correct the defect. In this case, the only thing that can save a sick child is a special prosthesis that is installed in place of the removed ventricle.
The problem of treating “blue” conotruncal defects in newborns is widely discussed, despite significant improvements in surgical strategy. The most common defects in this group are tetralogy of Fallot and pulmonary artery atresia (PA) in combination with a ventricular septal defect (VSD). Prematurity and low birth weight continue to be risk factors for poor results of traditional surgical interventions [3, 8, 12]. Attempts to delay surgery through supportive care result in increased morbidity and mortality [1]. In most uncomplicated cases, the optimal treatment method for patients with right ventricular outflow tract (RVOT) obstruction is radical correction of the defect at an early age. Currently, the results of radical correction of this group of conotruncal defects are successful in 90–96% of patients, including children under one year of age [6]. The possibility of its implementation depends on individual anatomical and physiological characteristics and the level of pulmonary blood flow. At the same time, there is a category of patients who still require staged treatment. One of the first stages of surgical treatment is the creation of systemic-pulmonary anastomoses. In case of failure of previously applied systemic-pulmonary anastomoses, it is preferable to perform palliative reconstruction of the outflow tract, which was done in this case. This operation gave good results in the immediate period, but after 3 months a complication developed - severe obstruction of the RVOT, accompanied by a significant deterioration in the child's condition.
Recently, in addition to abdominal interventions, it has become possible to use endovascular treatment methods that increase pulmonary blood flow [7, 11]. These methods include patent ductus arteriosus (PDA) stenting, balloon valvuloplasty, and RVOT stenting. For the first time, stent implantation in the RVOT was proposed and developed by Y. Almagor in 1990. Since 1992, there have been reports of RVOT stenting in a small number of adult children [5, 10].
The use of this method may be preferable in patients with low body weight and other associated anomalies with complex congenital heart defects. The goal of this method is to ensure sufficient levels of pulmonary blood flow [1].
We present a case of successful stenting of the RVOT for PLA in combination with VSD in a 6-month-old child with low body weight, performed for emergency reasons due to progressive hypoxemia and attacks of shortness of breath accompanied by cyanosis.
Patient P., 6 months old, body weight 3 kg, was re-hospitalized on November 15, 2012 for emergency indications in the pediatric cardiac surgery department of the Federal State Budgetary Institution Federal Center for Cardiovascular Surgery of the Ministry of Health of Russia (Penza) with shortness of breath, severe attacks of shortness of breath, accompanied by cyanosis up to 3 times a day. Previously, in the neonatal period, type I pulmonary atresia and VSD were diagnosed, and in our institution a central aortopulmonary anastomosis was created using a Gore-Tex 3.5 mm prosthesis. The early postoperative period was complicated by shunt thrombosis, and during the same hospitalization palliative reconstruction of the outflow tract from the right ventricle was performed with a good result. At a follow-up examination after 1 month, blood oxygen saturation was 91%. According to echocardiography (EchoCG): atrial septal defect (ASD) 6 mm, subaortic VSD 10 mm with bidirectional discharge, RVOT passage 9 mm, Vmax 4.4 m/s, Gmax 78 mmHg. (McGoon index 1.33, Nakata index 119).
At the next examination, 3 months later, the child’s condition worsened.
On admission on 11/15/12, according to echocardiography, progression of RVOT stenosis was noted with a decrease in the orifice to 2 mm, Vmax 4.76 m/s, Gmax 92 mmHg. Blood oxygen saturation was 70%, decreasing with anxiety to 50%. The severity of the child's condition was due to arterial hypoxemia, attacks of shortness of breath accompanied by cyanosis. In order to minimize surgical trauma, it was decided to implant a stent in the outflow tract of the right ventricle as the next stage of treatment.
On 11/16/12 the left jugular vein was punctured. A 5F introducer was installed. Heparin (100 IU/kg) was administered prophylactically once. A 5F JR guiding catheter (Launcher, Medtronic) was installed in the right ventricle (RV), right ventriculography was performed in 2 projections, which revealed a pronounced narrowing of the RV outflow tract. Two coronary hydrophilic guidewires (Wisper ES, Abbot) were passed into the distal parts of the right branch of the pulmonary artery. A 6.5-12 mm balloon-expandable bare metal stent (RX Herculink Elite, Abbot) is positioned (in lateral projection) in the outflow tract of the pancreas with exit to the middle section of the pulmonary artery trunk. Positioning control was carried out using manual injection of a contrast agent through a catheter. A stent was implanted under a pressure of 12 atm. According to control angiography, the stent is fully deployed. To increase the radial stability of the stent, taking into account the extent of the infundibular stenosis, it was decided to implant an additional stent. An RX Herculink Elite 7.0-18 mm stent was implanted, overlapping with the previous one (stent overlap was almost 50%) under a pressure of 14 atm. During control angiography: the stents are patent and fully extended (Fig. 1).
Figure 1. Postoperative right ventriculogram (lateral view).
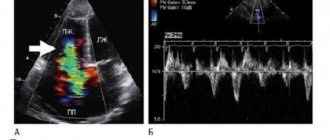
In the outflow tract of the right ventricle, stents with a sufficiently wide exit into the pulmonary artery are visualized. During the operation, no rhythm or conduction disturbances were observed. The total duration of fluoroscopy was 15 minutes. Blood oxygen saturation increased to 95%. Intraoperatively, according to echocardiography, a 20 mm long stent is visualized in the RVOT. The passage opening was 6 mm, Vmax 2.7 m/s, Gmax 29 mmHg. (see Fig. 2, color insert).
Figure 2. Postoperative echocardiograms. a — parasternal short-axis projection: blood flow through the stent into the outflow tract of the right ventricle is visualized, the dotted line indicates the length of the stent; b — Doppler study: peak gradient at the outflow tract of the right ventricle is 29 mm Hg. The early postoperative period was uneventful. The patient was extubated as planned on the 2nd day after surgery. Blood oxygen saturation remained at 96-100%.
In dynamics, after 5 days in the outflow tract of the pancreas, according to echocardiography, blood flow is carried out through the stent at Vmax 2.43 m/s, Gmax 24 mm Hg, insufficiency of degrees I-II. There was also an increase in the size of the pulmonary arteries (Nakata index 292, McGoon index 2.17).
On the 6th day, the child was transferred to a therapeutic hospital at the place of residence with a recommendation to take a prophylactic dose of aspirin 5 mg/kg/day.
Discussion
Thanks to the constant improvement of endovascular methods, reports of their use as an alternative to abdominal palliative operations have increasingly begun to appear [7, 11].
Thus, stent implantation provides an effective temporary alternative to surgical correction of RVOT obstruction. However, this tactic does not replace surgical treatment, but only postpones it until the period of somatic growth [1, 5].
Analysis of long-term results of the most widely used palliative endovascular interventions (stenting of a PDA, creation of a systemic-pulmonary shunt) demonstrated a number of problems. Thus, compared with stenting of the outflow tract of the pancreas, implantation of a stent in the PDA may lead to steal syndrome with a decrease in visceral perfusion associated with lower diastolic blood pressure. In addition, there is a high probability of neointimal hyperplasia inside the stent, and arterial vascular access is also required during implantation [2].
In our case, stenting of the PDA was not possible, since the duct was no longer functioning.
The creation of systemic-pulmonary anastomoses in almost 35% of cases is complicated by hypoplasia and deformation of the pulmonary artery, especially if the operation took place during the neonatal period [4].
Implantation of a stent in the RVOT is applicable for the palliative treatment of infundibular stenosis in the case of other anomalies when surgical correction is impossible or involves a high risk. The pulsating nature of antegrade blood flow through the pulmonary arteries is preferable, as it promotes its physiological growth, bringing closer the implementation of radical correction [1].
Potential limitations of this approach include stent obstructions due to neointimal hyperplasia or muscle proliferation, stent fractures, and the possibility of perforation of the pancreatic outflow tract [1, 5]. These complications can be minimized if, after stent implantation, the next stage of surgical treatment is performed in a timely manner.
The intervention can be technically complex and accompanied by possible complications such as incorrect position of the stent, stent collapse, migration of the stent into the pulmonary artery, which is associated with the implantation technique and certain imperfections of existing stents [1, 10]. The issue of calculations when choosing the size of a stent implanted in the pancreas and pulmonary artery has not been clearly resolved. L. Gibbs [5] considers it reasonable to implant the largest possible diameter of the stent to achieve maximum radial opening force and to ensure the possibility of postdilation in the future with an increase in the diameter of the infundibular region as the child grows, as well as compensation for neointimal hyperplasia. At the same time, the creation of excess pulmonary blood flow is extremely undesirable [6].
Nevertheless, this is a very promising method, and with the development of endovascular technologies and the advent of instruments with a small profile delivery system, it has become acceptable for newborns and premature patients.
Preliminary results from a small number of observations show that stent implantation for RVOT obstruction is an effective and often the only possible method that allows children in serious condition to wait for the next stage of treatment [1].