Compound
| Film-coated tablets | 1 table |
| active substance: | |
| atorvastatin calcium trihydrate | 10.85 mg |
| 21.7 mg | |
| (equivalent to 10 and 20 mg atorvastatin, respectively) | |
| excipients: calcium carbonate - 33/66 mg; MCC - 48/96 mg; lactose monohydrate (milk sugar) - 23.85/47.7 mg; pregelatinized starch (starch 1500) - 32.8/65.6 mg; colloidal silicon dioxide (Aerosil) - 0.75/1.5 mg; magnesium stearate - 0.75/1.5 mg; polyvinyl alcohol - 2.5/5 mg; macrogol (polyethylene glycol) - 1.26/2.52 mg; talc - 0.93/1.86 mg; titanium dioxide - 1.56/3.12 mg |
Contraindications
According to the instructions for use, it is recommended to avoid using the medication while expecting a baby and breastfeeding. The medicine should not be taken while planning pregnancy. It is contraindicated in patients under 18 years of age. The medication should not be taken if you are hypersensitive to its components. The drug is contraindicated in cases of lactose intolerance and glucose-galactose malabsorption.
The drug is used with caution in the presence of the following pathologies:
- arterial hypertension;
- epilepsy;
- diabetes;
- pathologies of the muscular system;
- water-electrolyte imbalance.
With long-term use of drugs from the group of statins, it is necessary to monitor liver function
Directions for use and doses
Inside.
Before prescribing Atorvastatin, the patient should be recommended a standard lipid-lowering diet, which he should continue to follow throughout the entire period of therapy.
The initial dose averages 10 mg/day. The dose varies from 10 to 80 mg/day.
The drug can be taken at any time of the day with food or regardless of meal time. The dose is adjusted based on baseline LDL/C cholesterol levels, goals of therapy, and individual response. At the beginning of treatment and/or during dose increases of Atorvastatin, plasma lipid levels should be monitored every 2–4 weeks and the dose adjusted accordingly.
Primary hypercholesterolemia and mixed hyperlipidemia, as well as Fredrickson types III and IV. In most cases, a dose of 10 mg of Atorvastatin once a day is sufficient. A significant therapeutic effect is observed after 2 weeks, and the maximum therapeutic effect is usually observed after 4 weeks. With long-term treatment, this effect persists.
Homozygous familial hypercholesterolemia. Prescribed at a dose of 80 mg (4 tablets of 20 mg each) 1 time per day.
Special patient groups
Renal dysfunction. The use of the drug in patients with renal failure and kidney disease does not affect the level of Atorvastatin in the blood plasma or the degree of reduction in cholesterol/LDL levels during its use, so a change in the dose of the drug is not required.
Liver dysfunction. In case of liver failure, the dose must be reduced.
Elderly patients. When using the drug in elderly patients, there were no differences in safety, effectiveness, or achievement of lipid-lowering therapy goals compared to the general population.
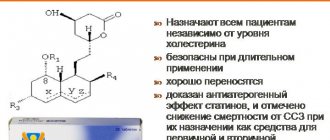
Therapeutic effect of the drug
Atorvastatin SZ tablets help reduce cholesterol in the blood. When using the medicine, the likelihood of the patient developing the following pathologies of the cardiovascular system is reduced:
- stroke;
- myocardial infarction.
The drug reduces the risk of complications of atherosclerosis in the presence of the following provoking factors:
- elderly age;
- presence of nicotine addiction;
- hypertonic disease;
- increased blood sugar concentration.
The drug Atorvastatin SZ helps reduce blood viscosity. It improves the condition of the hematopoietic system and prevents the rupture of atherosclerotic plaques.
The main task of atorvastatin is to reduce the amount of cholesterol in the human body.
Analogues of "Atorvastatin"
Currently, three generations of drugs are actively used to treat atherosclerosis, dyslipidemia and hypercholesterolemia. Modern analogues of Atorvastatin are the drugs Rosuvastatin, Simvastatin, Pravastatin, Fluvastatin and others. Depending on the manufacturer of the product, its trade name may also change.
The original drug with the active ingredient atorvastatin is called “Liprimar”. Its substitutes, or imported analogues, have the names: “Atoris”, “Tulip”, “Liptonorm”. Such drugs are generics - drugs under an international name, or under a patented name that differs from the original one, and is registered to another pharmaceutical developer (company). It should be borne in mind that such medications can be produced in different dosages and different numbers of tablets per package.
Since the emergence of information that the effect of statins in patients with an increased risk of myocardial infarction or stroke increases life expectancy, the question of choosing the most effective and safe drug from this group has become very relevant. The reason for this article was the publication of the results of the largely unique PROVE-IT study, as well as data obtained in the REVERSAL study.
Variety of statins
To date, a fairly large number of different statins have been developed. Lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin, cerivastatin and rosuvastatin have reached the stage of clinical use. Cerivastatin has been withdrawn due to safety concerns. These drugs inhibit 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), reducing the synthesis of mevalonate and, accordingly, all substances synthesized with its participation. The action of statins is based on their interaction with the active center of the enzyme. The most obvious and easily recorded result is a decrease in cholesterol (C) synthesis. All statins have a structure reminiscent of HMG-CoA, but more massive and hydrophobic. At the same time, there are significant differences among representatives of this group of drugs.
Statins are divided into 2 classes based on their structure. The first of these includes pravastatin and simvastatin. In addition to a structure similar to HMG-CoA, they contain a decalin ring. Rosuvastatin, atorvastatin, cerivastatin and fluvastatin belong to the second class and contain fluorophenyl and methylethyl groups [1].
Lovastatin, simvastatin and pravastatin are substances originally isolated from Aspergillus terreus, while lovastatin is a natural product (derivative of mevinic acid), while simvastatin and pravastatin are partially modified. Atorvastatin, fluvastatin, cerivastatin and rosuvastatin are completely synthetic drugs.
Lovastatin and simvastatin are prodrugs; the remaining statins are active forms.
The two statins being compared have a number of significant differences in pharmacokinetics (see table). Pravastatin is the most hydrophilic of the statins and significantly more hydrophilic than atorvastatin.
The fact that atorvastatin (like most other statins) is metabolized with the participation of the CYP3A4 isoenzyme indicates that when used together with other drugs metabolized by this cytochrome (cyclosporine, erythromycin, etc.), the risk of side effects may increase . About 47% of the most commonly used drugs are metabolized by CYP3A4.
Pravastatin
To date, one of the most studied statins is pravastatin (a naphthalene-heptanoic acid derivative). In 2003, the results of 28 studies were published, involving 57 thousand patients. In this indicator, pravastatin is superior only to simvastatin, in studies of which more than 78 thousand people took part.
Among studies of the effectiveness of pravastatin conducted in patients with coronary artery disease at high risk of an unfavorable outcome, the most significant is the LIPID (Long-term Intervention with Pravastatin in Ischemic Disease) study, which included 9014 patients aged 31–75 years [2]. At the same time, in 5754 patients the reason for inclusion was a previous myocardial infarction, in 3260 – unstable angina. Pravastatin 40 mg was used in comparison with placebo. The average follow-up period was 6.1 years. The main result of the study was a 24% reduction in the risk of death from coronary artery disease in the treatment group (p
In a study by Ishikawa K et al. [3], published in 2003, found that pravastatin significantly reduced the volume of atherosclerotic plaque measured by three-dimensional intravascular ultrasound after 6 months. The degree of plaque volume reduction was significantly correlated with changes in high-density lipoprotein (HDL) levels and did not correlate with changes in low-density lipoprotein (LDL) or total cholesterol levels.
Atorvastatin
Atorvastatin significantly reduces LDL levels to a greater extent. This is a completely synthetic statin - a derivative of pyrolysis-heptanoic acid. Compared to simvastatin and pravastatin, this drug has been studied somewhat less. The greatest resonance was caused by data showing that the effect of atorvastatin can occur quite quickly. The most revealing results were the results of the MIRACL study.
The MIRACL (Myocardial Ischaemia Reduction with Aggressive Cholesterol Lowering) study examined the effectiveness of atorvastatin compared with placebo in patients who had suffered an acute coronary syndrome (non-Q wave myocardial infarction, large focal myocardial infarction, or an episode of unstable angina). A total of 3086 patients participated in the study, with an average age of 65 ± 12 years [4]. Patients were observed for 16 weeks. As a result, it was found that the incidence of the so-called summary end point (death, non-fatal myocardial infarction, cardiac arrest with resuscitation, readmission due to recurrent ischemia) in the atorvastatin group was lower - 14.8 versus 17.4% in the placebo group, Moreover, the reliability of the difference turned out to be relatively low: p = 0.048. The reduction in the risk of readmission for recurrent ischemia was more significant: p = 0.02. The incidence of stroke in the atorvastine group decreased by 50%. However, the risk of death, nonfatal heart attack, or cardiac arrest did not significantly decrease during the study. The level of LDL cholesterol under the influence of atorvastatin fell statistically significantly by 40% (from 3.3 to 1.9 mmol/l). In addition, while taking atorvastastatin, a significant decrease in pro-inflammatory markers (C-reactive protein, serum amyloid type A) was noted. C-reactive protein during the use of atorvastatin by the 16th week of treatment decreased by 85% and was 34% less than in the placebo group [5]. In the atorvastatin group, a significantly more frequent increase in transaminase levels was noted (2.5 versus 0.6% , R
Thus, by 2000, although atorvastatin demonstrated a more pronounced lipid-lowering effect, pravastatin had more advantages, including potentially fewer interactions with other drugs, greater hydrophilicity and a significant number of controlled studies. There is a need to compare the effects of various statins, which was implemented in two studies, which will be discussed below.
REVERSAL Study
The REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study was conducted to compare the effect of 40 mg pravastatin and 80 mg atorvastatin per day on the dynamics of coronary atherosclerosis [6]. From June 1999 to September 2001, of 2163 patients screened, 654 patients were randomized to receive study drugs. The final analysis included 502 patients (249 in the pravastatin group and 253 in the atorvastatin group) who were able to perform intravascular ultrasound of satisfactory quality at baseline and after 18 months of treatment with study drugs. The main parameter assessed was the dynamics of the volume of coronary atheroma during the study. Baseline LDL level in both groups was 150.2 mg/dL (3.89 mol/L). It decreased to 110 mg/dL (2.85 mmol/L) in the pravastatin group and to 79 mg/dL (2.09 mmol/L) in the atorvastatin group. The significance of differences in lipid dynamics turned out to be highly significant (p
The degree of change in atheroma volume was significantly less during treatment with atorvastatin (p = 0.02). During the use of pravastatin, atherosclerotic lesions progressed (by 2.7%; p = 0.001), while there was no progress in the atorvastatin group over the same period.
The difference between the results of the REVERSAL study and the work of Ishikava K et al. [3] can be explained by at least 3 reasons. First, the work cited in the section on pravastatin was performed on a small number of patients; secondly, the duration of treatment with pravastatin is quite short (only 6 months versus 18 in the REVERSAL study); finally, thirdly, the work was carried out in Japan, and, as is known, the Japanese are significantly more sensitive to statin therapy.
PROVE-IT Study – TIMI-22
The PROV-IT - TIMI-22 study (Pravastatin or Atorvastatin Evaluation and Infection Therapy - Thrombolysis in Myocardial Infarction 22) was conducted to compare 2 statin regimens (and, in fact, compare 2 drugs prescribed in maximum dosages) [7]. Pravastatin at a dose of 40 mg/day was used as a drug that leads to a “standard” reduction in LDL levels below 100 mg/dL (2.59 mmol/L). Atorvastatin was used as a drug to more intensively lower LDL levels to 70 mg/dL (1.81 mmol/L).
From November 2000 to December 2001, the study randomized 4162 patients (men and women over the age of 18 years) hospitalized with acute coronary syndrome (both with and without ST-segment elevation) or who had an episode of unstable high-grade angina. risk within 10 days before inclusion. At the time of randomization, the level of total cholesterol should not exceed 240 mg/dl (6.21 mmol/l), if patients had not previously received statins. If statins were previously prescribed, the TC level should not exceed 200 mg/dL (5.18 mmol/L). Patients who had previously received statins at a dose of 80 mg, as well as those receiving drugs metabolized by CYP3A4, were not included in the study.
For acute coronary syndrome, patients received all necessary treatment, including acetylsalicylic acid, clopidogrel and warfarin. Patients were randomized 1:1 to treatment with atorastatin (80 mg/day) or pravastatin (40 mg/day). In addition, the same study examined the effectiveness of a month-long course of the antibacterial drug gatifloxacin (the results of this part of the protocol have not yet been published). Patients were followed up 30 days after inclusion, then every 4 months until August–September 2003, when the final visit was performed. The follow-up period was 18–36 months (average 24). After 925 endpoints were recorded, the study was stopped.
The endpoints of the study were death from any cause; myocardial infarction, documented unstable angina requiring hospitalization; revascularization (angioplasty or coronary artery bypass grafting) performed later than 30 days after randomization; stroke.
A total of 2063 patients were included in the pravastatin group and 2099 in the atorvastatin group. The groups were completely comparable in all main clinical characteristics, with the exception of the incidence of peripheral atherosclerosis, which was more often observed among patients included in the pravastatin group (p = 0.03).
From the 30th day of the study, a significant difference in LDL levels was noted between the groups (Fig. 2). During the study, the pravastatin group achieved an average LDL level of 95 mg/dL (2.46 mmol/L) and the atorvastatin group achieved an average LDL level of 62 mg/dL (1.60 mmol/L); differences between groups in the degree of LDL reduction were highly significant (p
There was also a significant decrease in the level of C-reactive protein from 12.3 mg/l in both groups to 2.1 mg/l in the pravastatin group and to 1.3 mg/l in the atorvastatin group. The differences in the degree of CRP reduction were highly significant.
The main result of the study was that during therapy with atorvastatin, the incidence of primary endpoints was 16% lower than in the pravastatin group (p = 0.005). At the same time, in the pravastatin group, the incidence of adverse outcomes during 2 years of observation was 26.3%, and in the atorvastatin group – 22.4% (Fig. 3).
Differences between groups began to be noted on day 30 of treatment and persisted throughout the study (Fig. 4).
If we isolate individual end points from the total risk, then during treatment with atorvastatin there was a significantly lower incidence of unstable angina requiring hospitalization (by 29%; p = 0.02), as well as the need for revascularization (by 14%; p = 0 ,04). Risk reductions, although not statistically significant, were observed for the risk of death from any cause (by 28%; p = 0.07) and the composite endpoint of death + myocardial infarction by 18% (p = 0.06). Stroke was rare in both groups and no significant difference was found.
The benefit of atorvastatin was greatest in patients with a baseline LDL level greater than 125 mg/dL (34% risk reduction), while in patients with a baseline LDL level the risk of the primary endpoint was reduced by only 7% (p = 0.02). In addition, the benefit of atorvastatin was more pronounced in patients who had not previously received statins.
The value of the data obtained in the PROVE-IT study lies in the fact that it was conducted by the pravastatin manufacturing company, whose specialists had sufficient reason to expect completely different results. The main hypothesis underlying the protocol was that there would be no difference between the two drugs, despite their different effects on lipids.
So, it should be stated that we have finally moved from comparisons with placebo to direct comparison of the effectiveness and safety of statins. The significance of the results already obtained consists of several points.
The recently published HPS study [8] specifically assessed sensitivity to a standard dose of simvastatin. It turned out that this indicator varies widely, and in 1/3 of patients after 6 weeks of therapy with simvastatin at a dose of 40 mg, LDL levels decreased by less than 38% from the initial level, in another 1/3 - by 38–48%, and for the rest - by more than 48%. At the same time, there were no differences in the degree of influence on the risk of adverse outcomes in these three groups. Thus, the question was raised whether the degree of LDL reduction could predict the effect of a statin on hard endpoints. The PROVE-IT study seems to answer this question in the affirmative. However, it should be remembered that this protocol did not compare two dosages of the same statin, but two different drugs.
An important question is whether these comparisons can be extrapolated to other statins. This primarily concerns rosuvastatin, a drug that leads to an even more pronounced decrease in proatherogenic fractions of blood lipoproteins. In our opinion, there are no grounds for this yet. First of all, it is necessary to ensure that other statins are similarly safe with the same reduction in LDL levels. In addition, direct comparisons are also necessary in order to definitively answer the question of whether the effect depends on the drug or only on the degree of impact on cholesterol metabolism.
Although in the PROVE-IT study the best effect was obtained with the use of a drug that has a more pronounced lipid-lowering effect, it is not possible to exclude the fact that the higher effectiveness of atorvastatin may be due to something else. Indeed, by blocking HMG-CoA reductase for a longer period than most other statins, atorvastatin reduces the synthesis of mevalonate and, accordingly, all products in the synthesis of which this substance takes part, for a longer period. In other words, the other consequences of blockade of mevalonate synthesis when using atorvastatin are more pronounced.
The question about the significance of the lipid-lowering effect in the action of statins could be answered by comparing the effectiveness of atorvastatin and the combination of simvastatin (or another statin) with ezetimibe in a similar protocol. This combination leads to a pronounced decrease in LDL levels, but the mechanism of this decrease is due not only to a decrease in the synthesis of mevalonate, but also to a blockade of the reabsorption of cholesterol in the small intestine. The same effectiveness of these interventions would indicate that the main mechanism of action of statins is the reduction of LDL levels, and everything else is just side effects that have no clinical significance.
A serious question is about the applicability of the data from the studies reviewed in everyday practice. According to their results, intensive statin therapy for acute coronary syndrome in patients admitted to hospital should be started as early as possible. Unfortunately, in our country, these data can be used with very significant restrictions. First, the availability of invasive interventions is very limited, while up to 60% of patients included in PROVE-IT had undergone such an intervention before the start of the study. In our population, many patients simply will not live to see the beneficial effects of statins. Secondly, the use of the 80 mg dosage of atorvastatin is limited by the lack of an appropriate dosage form in Russia.
It is impossible not to dwell on one more point. The data obtained in the PROVE-IT study are relevant to patients at highest risk of developing an unfavorable outcome of coronary artery disease—patients who have just experienced an exacerbation of coronary artery disease. Thus, another step has been taken in choosing the optimal management strategy for patients who have suffered a myocardial infarction or unstable angina.
Features of interaction with other medications
Atorvastatin SZ is not recommended for use simultaneously with Varvarin. A medicine intended to reduce cholesterol in the body interacts poorly with the following drugs:
- drugs with an antifungal effect;
- antibiotics from the macrolide group;
- drugs that have an immunosuppressive effect.
When using antacids intended to neutralize hydrochloric acid, the content of the active component of the drug in the plasma may decrease. If it is necessary to use such drugs simultaneously with Atorvastatin SZ, the decision to increase the dosage of the lipid-lowering drug should be made by the doctor.
Only a specialist should prescribe statins. Self-medication is dangerous for your health!
The shelf life of the medicine is three years. The medicine should be stored in a place protected from direct sunlight. The optimal temperature for storing the drug is 25 degrees.
How to enhance the effect of the medicine?
To enhance the therapeutic effect of the drug, it is recommended to adhere to a strict diet. Its main goal is to improve lipid metabolism.
If the level of cholesterol in the body is high, you need to limit the consumption of foods that contain animal fats. The diet includes dishes that saturate the body with fiber.
If you have high cholesterol, you should eat foods that contain large amounts of phytosterols. These include:
- sesame seeds;
- products containing wheat germ;
- sunflower seeds;
- flax seeds;
- olive oil;
- avocado;
- oil squeezed from grape seeds.
The diet should also include foods enriched with pectin: citrus fruits, apples, watermelons. If you have high cholesterol levels in your blood, the following foods are also helpful:
- spinach;
- green salad leaves;
- artichoke;
- sorrel;
- dill;
- parsley.
When using the drug Atorvastatin SZ, it is recommended to avoid eating pork, duck meat, offal, smoked meats, and sausages. Fatty fish, red caviar, shrimp, and canned fish are also prohibited.
It is recommended to exclude the following dishes from the diet:
- baked goods;
- mushroom broth;
- cream;
- high fat cottage cheese;
- sour cream;
- ice cream;
- chocolate products;
- palm oil products;
- mayonnaise;
- ketchup.
No ads 3
Side effects
When using the medicine, side effects from the nervous system may occur:
- pain in the head;
- dizziness;
- worsening sleep;
- the occurrence of asthenic syndrome;
- paresthesia;
- peripheral neuropathy.
When using the drug, the following side effects may also occur:
- the occurrence of tinnitus;
- cardiopalmus;
- increased blood pressure;
- arrhythmia;
- nosebleeds;
- thrombocytopenia;
- nausea;
- flatulence;
- pain in the abdominal area;
- deterioration of taste perception;
- decreased sex drive;
- the appearance of swelling in the joint area;
- muscle cramps;
- liver failure;
- hair loss;
- skin itching;
- peripheral edema;
- chest pain;
- lethargy;
- a veil before the eyes;
- memory impairment;
- increase in the concentration of glycosylated hemoglobin.
No ads 1