pharmachologic effect
Lipid-lowering agent - HMG-CoA reductase inhibitor
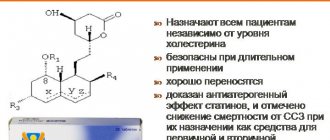
Atorvastatin is a selective competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), a key enzyme that converts 3-hydroxy-3-methylglutaryl-CoA to mevalonate, a precursor of sterols, including cholesterol.
In patients with homozygous and heterozygous familial hypercholesterolemia, non-familial forms of hypercholesterolemia and mixed dyslipidemia, atorvastatin reduces the plasma concentrations of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and apolipoprotein B (Apo-B), as well as lipoprotein cholesterol very low density lipoprotein cholesterol (VLDL-C) and triglycerides (TG), causes an unstable increase in the concentration of high-density lipoprotein cholesterol (HDL-C).
Atorvastatin reduces the concentration of cholesterol and lipoproteins in the blood plasma by inhibiting HMG-CoA reductase and cholesterol synthesis in the liver and increasing the number of “liver” LDL receptors on the cell surface, which leads to increased uptake and catabolism of LDL-C.
Atorvastatin reduces the formation of LDL-C and the number of LDL particles, causes a pronounced and persistent increase in the activity of LDL receptors in combination with favorable qualitative changes in LDL particles, and also reduces the concentration of LDL-C in patients with homozygous hereditary familial hypercholesterolemia, resistant to therapy with other lipid-lowering drugs means.
Atorvastatin in doses from 10 mg to 80 mg reduces the concentration of total cholesterol by 30-46%, LDL-C by 41-61%, Apo-B by 34-50% and TG by 14-33%. The results of therapy are similar in patients with heterozygous familial hypercholesterolemia, non-familial forms of hypercholesterolemia and mixed hyperlipidemia, including patients with type 2 diabetes mellitus.
In patients with isolated hypertriglyceridemia, atorvastatin reduces the concentration of total cholesterol, LDL-C, VLDL-C, Apo-B and TG and increases the concentration of HDL-C. In patients with dysbetalipoproteinemia, atorvastatin reduces the concentration of intermediate-density lipoprotein cholesterol. In patients with Fredrickson type IIa and IIb hyperlipoproteinemia, the mean increase in HDL-C concentrations during treatment with atorvastatin (10-80 mg) compared to baseline is 5.1-8.7% and is independent of dose. There is a significant dose-dependent decrease in the ratios: total cholesterol/HDL-C and LDL-C/HDL-C by 29-44% and 37-55%, respectively.
Atorvastatin at a dose of 80 mg significantly reduces the risk of ischemic complications and mortality by 16% after a 16-week course, and the risk of readmission for angina pectoris accompanied by signs of myocardial ischemia by 26%. In patients with different baseline LDL-C concentrations, atorvastatin causes a reduction in the risk of ischemic complications and mortality (in patients with non-Q wave myocardial infarction and unstable angina, as well as in men and women, and in patients younger and older than 65 years). The decrease in plasma LDL-C concentration correlates better with the dose of the drug than with its plasma concentration. The dose is selected taking into account the therapeutic effect (see section “Method of administration and dosage”). The therapeutic effect is achieved 2 weeks after the start of therapy, reaches a maximum after 4 weeks and persists throughout the entire period of therapy.
Atorvastatin 10 mg reduces fatal and nonfatal coronary artery disease (CHD) compared with placebo in hypertensive patients with three or more risk factors.
Indications for use
- primary hypercholesterolemia (heterozygous familial and non-familial hypercholesterolemia (type IIa according to the Fredrickson classification);
- combined (mixed) hyperlipidemia (types IIa and IIb according to the Fredrickson classification);
- dysbetalipoproteinemia (type III according to the Fredrickson classification) (as an addition to a hypocholesterol diet);
- familial endogenous hypertriglyceridemia (type IV according to the Fredrickson classification), resistant to a hypocholesterol diet;
- homozygous familial hypercholesterolemia with insufficient effectiveness of diet therapy and other non-pharmacological treatments;
- primary prevention of cardiovascular complications in patients without clinical signs of coronary heart disease, but with several risk factors for its development: age over 55 years, nicotine addiction,
- arterial hypertension, diabetes mellitus, low concentrations of HDL cholesterol in the blood plasma, genetic predisposition, including dyslipidemia;
- secondary prevention of cardiovascular complications in patients with coronary heart disease in order to reduce the total mortality rate, myocardial infarction, stroke, re-hospitalization for angina pectoris and the need for revascularization procedures.
Novostat instructions for use
Instructions for medical use of the drug Novostat
Compound
The 20.0 mg capsule contains the active substance: atorvastatin calcium trihydrate (crystalline) - 21.69 mg, in terms of atorvastatin - 20.0 mg.
Pharmacotherapeutic group
Lipid-lowering agent - HMG-CoA reductase inhibitor
ATX code S10AA05
pharmachologic effect
Synthetic lipid-lowering agent. Atorvastatin is a selective competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), a key enzyme that converts 3-hydroxy-3-methylglutaryl-CoA into mevalonate, a precursor of sterols, including cholesterol. In patients with homozygous and heterozygous familial hypercholesterolemia, non-familial forms of hypercholesterolemia and mixed dyslipidemia, atorvastatin reduces the plasma concentrations of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and apolipoprotein B (Apo-B), as well as lipoprotein cholesterol very low density lipoprotein cholesterol (VLDL cholesterol) and triglycerides (TG), causes an unstable increase in the concentration of high density lipoprotein cholesterol (HDL cholesterol). Atorvastatin reduces the concentration of cholesterol and lipoproteins in the blood plasma by inhibiting
HMG-CoA reductase and cholesterol synthesis in the liver and, increasing the number of “liver” LDL receptors on the cell surface, which leads to increased uptake and catabolism of LDL-C.
Atorvastatin reduces the formation of LDL-C and the number of LDL particles, causes a pronounced and persistent increase in the activity of LDL receptors in combination with favorable qualitative changes in LDL particles, and also reduces the concentration of LDL-C in patients with homozygous hereditary familial hypercholesterolemia, resistant to therapy with other lipid-lowering drugs means.
Indications for use
• Primary hypercholesterolemia (heterozygous familial and non-familial hypercholesterolemia (type IIa according to the Fredrickson classification);
• Combined (mixed) hyperlipidemia (types IIa and IIb according to the Fredrickson classification);
• Disbetalipoproteinemia (type III according to the Fredrickson classification) (as an addition to a hypocholesterol diet);
• Familial endogenous hypertriglyceridemia (type IV according to the Fredrickson classification), resistant to a hypocholesterol diet;
• Homozygous familial hypercholesterolemia with insufficient effectiveness of diet therapy and other non-pharmacological treatment methods;
• Primary prevention of cardiovascular complications in patients without clinical signs of coronary heart disease, but with several risk factors for its development: age over 55 years, nicotine addiction, arterial hypertension, diabetes mellitus, low concentrations of HDL-C in the blood plasma, genetic predisposition, including against the background of dyslipidemia;
• Secondary prevention of cardiovascular complications in patients with coronary artery disease to reduce the cumulative rate of mortality, myocardial infarction, stroke, readmission for angina and the need for revascularization procedures.
Contraindications
Hypersensitivity to any component of the drug. Active liver disease or an increase in the activity of “liver” transaminases in the blood plasma of unknown origin by more than 3 times compared with the upper limit of normal (ULN). Age under 18 years (insufficient clinical data on the effectiveness and safety of the drug in this age group).
Use in women of reproductive age who do not use adequate methods of contraception. Pregnancy, breastfeeding period. Lactose intolerance, lactase deficiency, glucose-galactose malabsorption syndrome.
Directions for use and doses
Inside. Take at any time of the day, regardless of meals. Before starting treatment with the drug, an attempt should be made to control hypercholesterolemia through diet, exercise and weight loss in obese patients, as well as treatment of the underlying disease. When prescribing the drug, the patient must be recommended a standard hypocholesterolemic diet, which he must adhere to during the entire period of therapy.
The dose of the drug varies from 10 mg to 80 mg 1 time per day and is titrated taking into account the initial level of LDL-C, the purpose of therapy and the individual effect on the therapy. The maximum daily dose of the drug for a single dose is 80 mg.
Release form
Capsules 10 mg, 20 mg, 40 mg and 80 mg. Together with instructions for use, it is placed in a cardboard package.
Conditions for dispensing from pharmacies
Dispensed by prescription.
Directions for use and doses
Inside. Take at any time of the day, regardless of meals. Before starting treatment with the drug, an attempt should be made to control hypercholesterolemia through diet, exercise and weight loss in obese patients, as well as treatment of the underlying disease. When prescribing the drug, the patient must be recommended a standard hypocholesterolemic diet, which he must adhere to during the entire period of therapy.
The dose of the drug varies from 10 mg to 80 mg 1 time per day and is titrated taking into account the initial level of LDL-C, the purpose of therapy and the individual effect on the therapy.
The maximum daily dose of the drug for a single dose is 80 mg.
At the beginning of treatment and/or while increasing the dose of the drug, it is necessary to monitor the concentration of lipids in the blood plasma every 2-4 weeks and adjust the dose of the drug accordingly.
- Primary hypercholesterolemia and combined (mixed) hyperlipidemia
For most patients - 10 mg 1 time per day; The therapeutic effect appears within 2 weeks of therapy and usually reaches a maximum within 4 weeks. With long-term treatment, the effect persists.
- Homozygous familial hypercholesterolemia
In most cases, 80 mg is prescribed once a day (reducing the concentration of LDL-C by 18-45%).
- Use in combination with other drugs
If necessary, simultaneous use with cyclosporine, the dose of the drug should not exceed 10 mg per day.
Caution should be used to use the lowest effective dose of atorvastatin when used concomitantly with HIV protease inhibitors, hepatitis C inhibitors, clarithromycin and itraconazole.
Atorvastatin Novostat caps 20 mg x30
ATX code: C10AA05 (Atorvastatin) Active substance: atorvastatin (atorvastatin) Rec.INN registered by WHO Dosage form NOVOSTAT caps. 20 mg: 10, 20, 30, 40, 50, 60, 80, 90, 100, 120, 150, 180, 200, 270 or 300 pcs. reg. No.: LP-002678 from 10/24/14 - Valid Release form, composition and packaging Hard gelatin capsules, No. 2, body and cap yellow with a light beige tint, opaque.
1 tab. atorvastatin calcium trihydrate 21.69 mg, which corresponds to the content of atorvastatin 20 mg
Excipients: lactose monohydrate - 87.41 mg, microcrystalline cellulose - 30 mg, sodium lauryl sulfate - 1 mg, povidone K17 - 5 mg, calcium carbonate - 35 mg, sodium carboxymethyl starch - 8 mg, magnesium stearate - 1.9 mg.
Composition of the capsule body: yellow iron oxide dye - 0.1%, titanium dioxide - 2%, gelatin - up to 100%. Composition of the capsule cap: yellow iron oxide dye - 0.1%, titanium dioxide - 2%, gelatin - up to 100%.
10 pieces. — cellular contour packages (1) — cardboard packs. 10 pieces. — contour cell packaging (2) — cardboard packs. 10 pieces. — cellular contour packages (3) — cardboard packs. 10 pieces. — contour cell packaging (4) — cardboard packs. 10 pieces. — contour cell packaging (5) — cardboard packs. 10 pieces. — contour cell packaging (6) — cardboard packs. 10 pieces. — contour cell packaging (9) — cardboard packs. 10 pieces. — contour cell packaging (10) — cardboard packs. 20 pcs. — cellular contour packages (1) — cardboard packs. 20 pcs. — contour cell packaging (2) — cardboard packs. 20 pcs. — cellular contour packages (3) — cardboard packs. 20 pcs. — contour cell packaging (4) — cardboard packs. 20 pcs. — contour cell packaging (5) — cardboard packs. 20 pcs. — contour cell packaging (6) — cardboard packs. 20 pcs. — contour cell packaging (9) — cardboard packs. 20 pcs. — contour cell packaging (10) — cardboard packs. 30 pcs. — cellular contour packages (1) — cardboard packs. 30 pcs. — contour cell packaging (2) — cardboard packs. 30 pcs. — cellular contour packages (3) — cardboard packs. 30 pcs. — contour cell packaging (4) — cardboard packs. 30 pcs. — contour cell packaging (5) — cardboard packs. 30 pcs. — contour cell packaging (6) — cardboard packs. 30 pcs. — contour cell packaging (9) — cardboard packs. 30 pcs. — contour cell packaging (10) — cardboard packs. 10 pieces. — polymer jars (1) — cardboard packs. 20 pcs. — polymer jars (1) — cardboard packs. 30 pcs. — polymer jars (1) — cardboard packs. 40 pcs. — polymer jars (1) — cardboard packs. 50 pcs. — polymer jars (1) — cardboard packs. 60 pcs. — polymer jars (1) — cardboard packs. 100 pieces. — polymer jars (1) — cardboard packs.
Clinical and pharmacological group: Lipid-lowering drug Pharmaco-therapeutic group: Lipid-lowering drug - HMG-CoA reductase inhibitor
Indications Primary hypercholesterolemia with ineffective diet therapy, combined hypercholesterolemia and hypertriglyceridemia, heterozygous and homozygous familial hypercholesterolemia with ineffective diet therapy. ICD-10 codes ICD-10 code Indication E78.0 Pure hypercholesterolemia E78.1 Pure hyperglyceridemia E78.2 Mixed hyperlipidemia
Dosage regimen Treatment is carried out against the background of a standard diet for patients with hypercholesterolemia. The dose is set individually, depending on the initial cholesterol level. Taken orally. The initial dose is usually 10 mg 1 time / day. The effect appears within 2 weeks, and the maximum effect occurs within 4 weeks. If necessary, the dose can be gradually increased at intervals of 4 weeks or more. The maximum daily dose is 80 mg. Side effects From the nervous system: > 1% - insomnia, dizziness, From the sensory organs: From the cardiovascular system: > 1% - chest pain, From the hematopoietic system: From the respiratory system: > 1% - bronchitis , rhinitis, From the digestive system: > 1% - nausea, From the musculoskeletal system: > 1% - arthritis, From the genitourinary system: > 1% - urogenital infections, peripheral edema, Dermatological reactions: > 1% - alopecia , xeroderma, photosensitivity, increased sweating, eczema, seborrhea, ecchymosis, petechiae.
From the endocrine system: From the metabolic side: Allergic reactions: Laboratory indicators: Contraindications for use Liver diseases in the active stage, increased activity of serum transaminases by more than 3 times of unknown origin, pregnancy, lactation (breastfeeding), women of reproductive age, not using reliable contraception, increased sensitivity to atorvastatin. Use during pregnancy and breastfeeding Atorvastatin is contraindicated for use during pregnancy and lactation (breastfeeding).
It is not known whether atorvastatin is excreted in breast milk. Considering the possibility of adverse events in infants, if it is necessary to use the drug during lactation, the issue of stopping breastfeeding should be decided.
Women of reproductive age should use adequate contraception during treatment. Atorvastatin should only be used in women of reproductive age if the likelihood of pregnancy is very low and the patient is informed of the possible risk of treatment to the fetus.
Use for liver dysfunction Contraindication: liver disease in the active stage.
Liver function tests should be monitored before and during treatment with atorvastatin, especially if symptoms of liver damage occur. If the level of transaminases increases, their activity should be monitored until normalization. If AST or ALT activity, more than 3 times higher than normal, persists, a dose reduction or discontinuation of atorvastatin is recommended.
Use in children In children, experience with the use of atorvastatin at doses up to 80 mg/day is limited. Special instructions Before and during treatment with atorvastatin, especially when symptoms of liver damage appear, liver function tests should be monitored. If the level of transaminases increases, their activity should be monitored until normalization. If AST or ALT activity, more than 3 times higher than normal, persists, a dose reduction or discontinuation of atorvastatin is recommended.
If symptoms of myopathy appear during treatment, CPK activity should be determined. If a significant increase in CPK levels persists, it is recommended to reduce the dose or discontinue atorvastatin.
The risk of myopathy during treatment with atorvastatin increases with concomitant use of cyclosporine, fibrates, erythromycin, azole antifungals, and niacin.
There is a possibility of developing the following adverse reactions, but not in all cases a clear connection has been established with taking atorvastatin: muscle cramps, myositis, myopathy, paresthesia, peripheral neuropathy, pancreatitis, hepatitis, cholestatic jaundice, anorexia, vomiting, alopecia, itching, rash, impotence, hyperglycemia and hypoglycemia.
In children, experience with the use of atorvastatin at doses up to 80 mg/day is limited.
Atorvastatin should be used with caution in patients with chronic alcoholism.
Drug interactions When atorvastatin is used simultaneously with digoxin, the concentration of digoxin in the blood plasma slightly increases.
Diltiazem, verapamil, isradipine inhibit the CYP3A4 isoenzyme, which is involved in the metabolism of atorvastatin, therefore, when used simultaneously with these calcium channel blockers, it is possible to increase the concentration of atorvastatin in the blood plasma and increase the risk of developing myopathy.
With simultaneous use of itraconazole, the concentration of atorvastatin in the blood plasma increases significantly, apparently due to the inhibition of its metabolism in the liver by itraconazole, which occurs with the participation of the CYP3A4 isoenzyme, increasing the risk of developing myopathy.
With simultaneous use of colestipol, it is possible to reduce the concentration of atorvastatin in the blood plasma, while the lipid-lowering effect is enhanced.
When used concomitantly, antacids containing magnesium hydroxide and aluminum hydroxide reduce the concentration of atorvastatin by approximately 35%.
With the simultaneous use of cyclosporine, fibrates (including gemfibrozil), antifungal drugs of azole derivatives, nicotinic acid, the risk of developing myopathy increases.
With simultaneous use of erythromycin and clarithromycin, the plasma concentration of atorvastatin moderately increases and the risk of developing myopathy increases.
With the simultaneous use of ethinyl estradiol and norethisterone (norethindrone), the concentration of ethinyl estradiol, norethisterone and (norethindrone) in the blood plasma increases slightly.
With simultaneous use of protease inhibitors, the concentration of atorvastatin in the blood plasma increases, because Protease inhibitors are inhibitors of the CYP3A4 isoenzyme.
Contraindications
- Hypersensitivity to any component of the drug.
- Active liver disease or an increase in the activity of “liver” transaminases in the blood plasma of unknown origin by more than 3 times compared with the upper limit of normal (ULN).
- Age under 18 years (insufficient clinical data on the effectiveness and safety of the drug in this age group).
- Use in women of reproductive age who do not use adequate methods of contraception.
- Pregnancy, breastfeeding period.
- Lactose intolerance, lactase deficiency, glucose-galactose malabsorption syndrome.
special instructions
Before starting therapy with Novostat, the patient must be prescribed a standard cholesterol-lowering diet, which he must follow during the entire treatment period.
The use of HMG-CoA reductase inhibitors to reduce the concentration of lipids in the blood can lead to changes in biochemical parameters reflecting liver function. Liver function should be monitored before starting therapy, 6 weeks, 12 weeks after starting Novostat and after each dose increase, and periodically, for example, every 6 months. An increase in the activity of liver enzymes in the blood serum may be observed during Novostat therapy.
Patients who experience increased enzyme activity should be monitored until enzyme activity returns to normal. In case of persistent increase in ALT or AST activity to a level exceeding 3 times the ULN, it is recommended to reduce the Novostat dose or discontinue treatment.
Novostat should be used with caution in patients who abuse alcohol and/or have liver disease. Active liver disease or persistent elevation of aminotransferase activity of unknown origin is a contraindication to the use of Novostat.
Treatment with Novostat, like other HMG-CoA reductase inhibitors, can cause myopathy. The diagnosis of myopathy (muscle pain and weakness in combination with an increase in CPK activity more than 10 times the ULN) should be discussed in patients with widespread myalgia, muscle soreness or weakness, and/or a marked increase in CPK activity. Patients should be warned to immediately report unexplained muscle pain or weakness if accompanied by malaise or fever. Novostat therapy should be discontinued in the event of a marked increase in CPK activity or in the presence of confirmed or suspected myopathy. The risk of myopathy during treatment with other drugs of this class increased with simultaneous use of cyclosporine, fibrates, erythromycin, nicotinic acid in lipid-lowering doses (more than 1 g/day) or azole antifungals. Many of these drugs inhibit cytochrome P4503A4-mediated metabolism and/or drug transport. Atorvastatin is biotransformed under the action of the CYP 3A4 isoenzyme.
When prescribing Novostat in combination with fibrates, erythromycin, immunosuppressive agents, azole antifungals or nicotinic acid in lipid-lowering doses (more than 1 g / day), the expected benefits and risks of treatment should be carefully weighed and patients should be regularly monitored to detect muscle pain or weakness, especially during the first months of treatment and during the period of increasing the dose of any drug. In such situations, periodic determination of CPK activity can be recommended, although such monitoring does not prevent the development of severe myopathy.
When using Novostat, as well as other drugs of this class, cases of rhabdomyolysis with acute renal failure caused by myoglobinuria have been described. Novostat therapy should be temporarily discontinued or completely discontinued if signs of possible myopathy occur or if there is a risk factor for the development of renal failure due to rhabdomyolysis (for example, severe acute infection, hypotension, major surgery, trauma, severe metabolic, endocrine and electrolyte disturbances and uncontrolled seizures ).
Before starting therapy with Novostat, an attempt should be made to control hypercholesterolemia through adequate diet therapy, increased physical activity, weight loss in obese patients, and treatment of other conditions. Patients should be warned to seek immediate medical attention if they experience unexplained muscle pain or weakness, especially if accompanied by malaise or fever.
When using HMG-CoA reductase inhibitors (statins), including atorvastatin, there have been cases of increased glycosylated hemoglobin and fasting plasma glucose concentrations. However, the risk of hyperglycemia is lower than the degree of reduction in the risk of vascular complications with statins.
There have been no reports of adverse effects of atorvastatin on the ability to drive vehicles and engage in potentially hazardous activities that require increased concentration and psychomotor speed. However, given the possibility of dizziness, caution should be exercised when performing these activities.
Novostat
The risk of myopathy during treatment with HMG-CoA reductase inhibitors increases with simultaneous use of cyclosporine, fibrates, erythromycin, clarithromycin, azole derivative antifungals, and nicotinic acid in lipid-lowering doses.
Inhibitors of the CYP3A4 isoenzyme: since atorvastatin is metabolized by the CYP3A4 isoenzyme, simultaneous use of atorvastatin with inhibitors of the CYP3A4 isoenzyme may lead to an increase in the concentration of atorvastatin in the blood plasma. The degree of interaction and potentiation effect are determined by the variability of the effect on the CYP3A4 isoenzyme. The simultaneous use of atorvastatin with drugs that reduce the concentration of endogenous steroid hormones (including cimetidine, ketoconazole, spironolactone) increases the risk of a decrease in endogenous steroid hormones.
OATP1B1 transport protein inhibitors: Atorvastatin and its metabolites are substrates of the OATP1B1 transport protein. OATP1B1 inhibitors (eg, cyclosporine) may increase the bioavailability of atorvastatin.
Erythromycin/clarithromycin: with simultaneous use of atorvastatin and erythromycin (500 mg 4 times a day) or clarithromycin (500 mg 2 times a day), which inhibit the CYP3A4 isoenzyme, an increase in the concentration of atorvastatin in the blood plasma was observed.
HIV protease inhibitors: simultaneous use of HIV protease inhibitors (fosamprenavir, nelfinavir, lopinavir/ritonavir combination, saquinavir/ritonavir, darunavir/ritonavir, fosamprenavir/ritonavir) with atorvastatin leads to an increase in the concentration of atorvastatin in the blood plasma.
Hepatitis C protease inhibitors: hepatitis C protease inhibitors (boceprevir), when used simultaneously with atorvastatin, lead to an increase in the concentration of atorvastatin in the blood plasma.
Diltiazem: Concomitant use of atorvastatin at a dose of 40 mg with diltiazem at a dose of 240 mg leads to an increase in the concentration of atorvastatin in the blood plasma.
Cimetidine: No clinically significant interaction was found.
Itraconazole: Concomitant use of atorvastatin at doses of 20 mg to 40 mg and itraconazole at a dose of 200 mg resulted in an increase in the AUC value of atorvastatin.
Grapefruit juice: Because grapefruit juice contains one or more components that inhibit CYP3A4, excessive consumption (more than 1.2 L per day) may cause an increase in plasma concentrations of atorvastatin.
Inducers of the cytochrome CYP3A4 isoenzyme: combined use of atorvastatin with inducers of the CYP3A4 isoenzyme (for example, efavirenz, rifampicin, phenytoin) may lead to a decrease in the concentration of atorvastatin in the blood plasma. Due to the dual mechanism of interaction with rifampicin (an inducer of the CYP3A4 isoenzyme and an inhibitor of the hepatocyte transport protein OATP1B1), simultaneous use of atorvastatin and rifampicin is recommended, since delayed administration of atorvastatin after taking rifampicin leads to a significant decrease in the concentration of atorvastatin in the blood plasma.
Antacids: simultaneous oral administration of a suspension containing magnesium hydroxide and aluminum hydroxide reduced the concentration of atorvastatin in the blood plasma, but the degree of reduction in the concentration of LDL-C did not change.
Phenazone: Atorvastatin does not affect the pharmacokinetics of phenazone, so interaction with other drugs metabolized by the same cytochrome isoenzymes is not expected.
Colestipol: with simultaneous use of colestipol, the concentration of atorvastatin in the blood plasma decreased, but the lipid-lowering effect of the combination of atorvastatin and colestipol was superior to that of each drug alone.
Digoxin: when using digoxin in combination with atorvastatin at a dose of 80 mg/day. digoxin concentration increased by approximately 20%. Patients receiving digoxin in combination with atorvastatin require appropriate monitoring.
Azithromycin: does not affect the plasma concentration of atorvastatin.
Oral contraceptives: When atorvastatin was coadministered with an oral contraceptive containing norethisterone and ethinyl estradiol, significant increases in the AUC of norethisterone and ethinyl estradiol by approximately 30% and 20%, respectively, were observed. This effect should be taken into account when choosing an oral contraceptive for a woman taking atorvastatin.
Terfenadine: with simultaneous use of atorvastatin and terfenadine, no clinically significant changes in the pharmacokinetics of terfenadine were detected.
Warfarin: There was no evidence of a clinically significant interaction between atorvastatin and warfarin.
Amlodipine: does not affect the pharmacokinetics of atorvastatin at steady state.
Fusidic acid: When atorvastatin is used concomitantly with fusidic acid, the risk of rhabdomyolysis increases. With their simultaneous use, the concentration of both fusidic acid and atorvastatin in the blood plasma may be increased. The mechanism of this interaction is still unknown. If treatment with fusidic acid is necessary, treatment with atorvastatin should be discontinued.
Colchicine: Caution should be exercised when atorvastatin is used concomitantly with colchicine, as cases of myopathy have been described.