- What is acidosis
- Reasons for development
- Types of acidosis
- Gas acidosis
- Non-gas acidosis
- Mixed acidosis
- Symptoms of acidosis
- Diagnosis of acidosis
- Treatment
Blood acidification is a very common phenomenon of our time.
This is due to the impact of harmful external factors of modern lifestyle and ecology. Violation of the acid-base balance in any direction, either acidification (Acidosis) or alkalization (Alkalosis), leads to a failure of vital systems. The acid-base state is assessed by the value of the hydrogen index (pH). From the point of view of physiology, the hydrogen indicators of the internal environments of the body, such as blood, intercellular fluid, are a rigid constant, because the slightest shifts disrupt the functioning of enzymes, and therefore lead to diseases, and with deviations of 0.5 in any direction, death is likely.
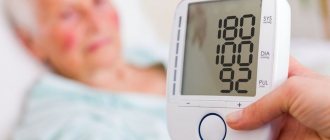
The normal range of blood pH is 7.35-7.45
What is Acidosis, what is the cause and how to deal with it?
Acidosis is a form of acid-base imbalance characterized by an absolute or relative excess of acids in the body.
Normally, the human body produces almost 20 times more acidic products than alkaline ones; therefore, systems that neutralize acidic compounds dominate in the body.
One of the reasons for a decrease in pH in the body is oxidative stress, the process of damage to cells and tissues by reactive oxygen species.
In the area of cell damage by reactive oxygen species, metabolic disturbances occur, inflammation develops, lactic, malic, succinic, a-ketoglutaric acids, under-oxidized products of lipolysis and proteolysis (fatty acids, polypeptides, amino acids, ketone bodies) accumulate.
The accumulation of acidic metabolites underlies the development of metabolic acidosis in the inflammatory zone. Moreover, the more intense the inflammation, the deeper the shifts in the acid-base state at the site of damage.
In the blood with acidosis, there is a decrease in pH below normal (below the average Ph value, taken as 7.39)
CONTENT
- 1 Signs and symptoms 1.1 Acute metabolic acidosis
- 1.2 Chronic metabolic acidosis
- 2.1 Reasons
- 3.1 Compensation mechanisms
- 4.1 Acute metabolic acidosis
- 5.1 Acute metabolic acidosis
Causes of acidosis
- Endogenous causes:
the most common and significant in clinical practice. In many life disorders, the functions of buffer systems and mechanisms for maintaining an optimal acid-base state for the body are disrupted - Exogenous causes:
associated with excessive intake of acidic substances into the body. For example, violation of the dosage of medications, the entry of toxic substances into the body. People who use synthetic diets (amino acids with acidic properties) often develop acidosis.
Prevention of diabetic ketoacidosis
Nothing super complicated or supernatural is required from you. You just need to be more careful about yourself and your health:
- measure your sugar more often and try to keep it within your target
- check ketones with visual test strips a couple of times a week
- Eat carbohydrates with every meal (if you have type 2, ask your doctor YOUR carbohydrate allowance per meal)
- improve your insulin injection and infusion technique
- do not overuse infusion sets
- When changing your infusion set, check your blood sugar 2-3 hours after installation to make sure insulin is reaching your body.
- do not change your infusion before bed
- minimize errors in calculating carbohydrates and dosages
- Do not skip insulin injections or take glucose-lowering medications!
Let us remember that ketoacidosis is a potentially dangerous, life-threatening condition.
Take care of yourself, Your DiaMarka!
Gas acidosis
It is characterized by a change in the content of carbon dioxide in the body, and as a result, an increase in the concentration of carbonic acid.
The main reason is a violation of alveolar ventilation of the lungs (during spasm or blockage of the airways) above the body's gas exchange needs for a certain time.
Characteristic manifestations are:
- Spasm of small bronchi and bronchioles, increased intracranial pressure
- Impairment of microcirculation and blood supply to tissues, which leads to the development of cell hypoxia, metabolic disorders and the formation of ATP (the body’s universal source of energy)
- Ion imbalance
Scientists have found that patients with chronic obstructive pulmonary disease are more likely to develop respiratory acidosis (COPD is known to develop due to oxidative stress, as free radicals cause damage to the alveoli)
Treatment of lactic acidosis
- Hemodialysis - infusion therapy is used to remove accumulated lactate from the cardiovascular system. Intravenous glucose and insulin injections trigger enzyme and protein activity.
- Artificial pulmonary ventilation (ALV) with intubation of the patient restores gas exchange, maintains impaired respiratory function, and reduces the concentration of carbon dioxide in the blood to normal levels. The procedure is carried out until the alkaline balance is restored.
- Cardiotonic drugs are prescribed to stimulate the activity of the heart muscle and normalize heart rhythm.
- Insulin therapy with prolonged effect.
As an emergency aid, the patient is administered sodium bicarbonate with a concentration of 4% or 2.5%, the daily dose is up to 2 liters. The drug is administered as a slow intravenous infusion, with adequate ventilation and calcium supplementation to mitigate side effects.
For patients with septic shock, crystalloid solutions - saline or saline - are prescribed in an amount of 30 mg/kg for the first 3 hours. Broad-spectrum antibiotics are used to combat concomitant infections.
Metformin
In case of lactic acidosis, the prescription of metformin to patients with type 2 diabetes is associated with the risk of complications due to possible overdose.
If metabolic acidosis is suspected, metformin should be stopped immediately and the patient should be hospitalized. It is important to preserve the vital functions of the body by stabilizing blood circulation and ensuring complete saturation of blood and tissues with oxygen. There is no specific antidote for metformin. In case of overdose, the introduction of activated carbon for adsorption may be indicated after a few hours.
Non-gas acidosis.
The main causes are metabolic disorders resulting from the development of oxidative stress, directly related to the increased formation of free radicals and a decrease in the effectiveness of the antioxidant system.
The most characteristic manifestations of non-gas acidosis are: decreased blood flow in the brain, kidneys and myocardium, edema, increasing depression of the nervous system (this is manifested by drowsiness, lethargy, and in severe cases - coma, for example in patients with diabetes mellitus)
Non-gas acid-base disorders (ABS) are, in turn, divided into three types: metabolic, excretory and exogenous.
Links[edit]
- Jaber, Samir; Pogam, Catherine; Foutier, Emmanuel; Lefran, Jean-Yves; Lasocki, Sigismond; Lesko, Thomas; Pottecher, Julien; Demoule, Alexander; Ferrandier, Martine; Asehnoun, Karim; Dellamonica, Jean; Valley, Lionel; Abbak, Paer Selim; Yong, Audrey de; Bruno, Vincent; Belafia, Fouad; Roquilli, Antoine; Schank, Gerald; Muller, Laurent; Constantin, Jean-Michel; Bertet, Elena; Klouche, Kada; Molinari, Nicholas; Jung, Boris; Jaber, Samir; Yong, Audrey de; Belafia, Fouad; Schank, Gerald; Monnin, Marion; Delay, Jean-Marc; Cisse, Moussa; Geniez, Marie; Conseil, Mathieu; Suchet, Bruno; Pogam, Catherine; Abbak, Paer Selim; Foutier, Emmanuel; Constantin, Jean Michel; Lefran, Jean-Yves; Muller, Laurent; Lasocki, Sigismond; Lesko, Thomas; Pottecher, Julien; Noll, Eric; Demoule, Alexander; Moravec, Eliza; Ferrandier, Martine; Acehnoun, Karim;Rokilly, Antoine; Dellamonica, Jean; Robert, Alexander; Valley, Lionel; Triglia, Thibault; Bruno, Vincent; Molinari, Nicholas; Mechati, Malika; Arnal, Jean-Michel; Durand-Gasselin, Jacques; Demoly, Didier; Graieh, Sami; Papazian, Laurent; Gilles, Vincent; Rimmele, Thomas; Rioux, Beatrice; Cougot, Pierre; Fourcade, Olivier; Seguin, Philippe; Charbit, Jonathan; Capdevila, Xavier; Leone, Mark; Zeleskevich, Laurent; Ichai, Carol; Orban, Jean Christophe; Darmon, Michael; Azoulay, Eli; Lemayel, Virginia; Zafrani, Lara; Debbat, Karim; Mimosa, Oliver; Guerin, Claude; Kipnis, Eric (July 7, 2021).Thomas; Rioux, Beatrice; Cougot, Pierre; Fourcade, Olivier; Seguin, Philippe; Charbit, Jonathan; Capdevila, Xavier; Leone, Mark; Zeleskevich, Laurent; Ichai, Carol; Orban, Jean Christophe; Darmon, Michael; Azoulay, Eli; Lemayel, Virginia; Zafrani, Lara; Debbat, Karim; Mimosa, Oliver; Guerin, Claude; Kipnis, Eric (July 7, 2021).Thomas; Rioux, Beatrice; Cougot, Pierre; Fourcade, Olivier; Seguin, Philippe; Charbit, Jonathan; Capdevila, Xavier; Leone, Mark; Zeleskevich, Laurent; Ichai, Carol; Orban, Jean Christophe; Darmon, Michael; Azoulay, Eli; Lemayel, Virginia; Zafrani, Lara; Debbat, Karim; Mimosa, Oliver; Guerin, Claude; Kipnis, Eric (July 7, 2021). "Sodium Bicarbonate Therapy for Patients with Severe Metabolic Acidemia in the Intensive Care Unit (BICAR-ICU): A Phase 3 Multicenter, Open-Lab, Randomized Controlled Trial" (PDF). Lancet
.
392
(10141):31–40. DOI: 10.1016/S0140-6736(18)31080-8. PMID 29910040. S2CID 49276138. - Navaneethan, Sankar D.; Shao, Jun; Buyss, Jerry; Bushinsky, David A. (July 5, 2021). "Effects of treatment for metabolic acidosis in CKD: a systematic review and meta-analysis". Clinical Journal of the American Society of Nephrology
.
14
(7): 1011–1020. DOI: 10.2215/CJN.13091118. PMC 6625635. PMID 31196951. - Kraut, Jeffrey A.; Madias, Nikolaos E. (4 September 2012). "Treatment of acute metabolic acidosis: a pathophysiological approach." Nature Reviews Nephrology
.
8
(10): 589–601. DOI: 10.1038/nrneph.2012.186. PMID 22945490. S2CID 34657707. - Jung, Boris; Rimmele, Thomas; Le Goff, Charlotte; Schank, Gerald; Korn, Philip; Jonquet, Olivier; Muller, Laurent; Lefran, Jean-Yves; Guervilly, Christophe; Papazian, Laurent; Allausic, Bernard; Jaber, Samir (2011). “Severe metabolic or mixed acidemia on admission to the intensive care unit: frequency, prognosis and administration of buffer therapy. Prospective multicenter study". Critical Care (London, England)
.
15
(5):R238. DOI: 10.1186/cc10487. PMC 3334789. PMID 21995879. - Inker, Leslie A.; Koresh, Joseph; Levy, Andrew S.; Tonelli, Marcello; Muntner, Paul (1 December 2011). "Estimated GFR, albuminuria, and complications of chronic kidney disease". Journal of the American Society of Nephrology
.
22
(12):2322–2331. DOI: 10.1681/ASN.2010111181. PMC 3279937. PMID 21965377. - ^ a b c
Emmett, Michael; Zerlip, Harold. "Approach to an adult with metabolic acidosis". - Costanzo, Linda (2010). Physiology
. Philadelphia, PA: Elsevier. ISBN 978-1-4160-6216-5. - ^ a b
Kraut, Jeffrey A.;
Madias, Nikolaos E. (05/01/2010). "Metabolic acidosis: pathophysiology, diagnosis and treatment." Nature Reviews Nephrology
.
6
(5): 274–285. DOI: 10.1038/nrneph.2010.33. ISSN 1759-5061. PMID 20308999. S2CID 205512465. - Gallo de Moraes, Alice; Surani, Salim (2019-01-15). "Effects of diabetic ketoacidosis on the respiratory system". World Journal of Diabetes
.
10
(1): 16–22. DOI: 10.4239/wjd.v10.i1.16. ISSN 1948-9358. PMC 6347653. PMID 30697367. - ^ a b
"National Kidney Foundation: K/DOQI Clinical Guidelines on Bone Metabolism and Disease in Chronic Kidney Disease" (PDF).
Am J Kidney Dis
. 42(Suppl 3): S1–S201. - ^ a b
"CKD Assessment and Management - KDIGO".
kdigo.org
. Retrieved December 31, 2021. - Kovesdy, Csaba. "Pathogenesis, consequences and treatment of metabolic acidosis in chronic kidney disease". UpToDate
. - Stern, Scott, D.C.; Cifu, Adam S.; Altcorn, Diane (2015). Symptom to Diagnosis: An Evidence-Based Guide
(3rd ed.). New York: McGraw-Hill Education. ISBN 9780071803441. OCLC 896866189. - Quinn, Jean R.; Gleason, Nathaniel W.; Papadakis, Maxine A.; McPhee, Stephen J., ed. (2016). Current manual of medical diagnosis and treatment
(2nd ed.). New York: McGraw-Hill. ISBN 9780071848053. OCLC 910475681. - ^ a b DeGowin's Diagnostic Examination
. LeBlond, Richard F., Brown, Donald D., 1940-, Suneja, Manish, Soth, Joseph F. (Tenth ed.). NY. 2014-09-05. ISBN 9780071814478. OCLC 876336892.CS1 maint: others (link) - Clinical anesthesiology by Morgan and Michael
. Butterworth, John F., IV, McKee, David K., Wasnik, John D., Morgan, J. Edward, Mikhail, Maged S., Morgan, J. Edward. (Sixth ed.). NY. 2018-08-21. ISBN 9781259834424. OCLC 1039081701.CS1 maint: others (link) - Poisoning and drug overdose
. Olson, Kent R. (Kent Russell), Anderson, Ilene B., Benowitz, Neil L., Blanc, Paul D., 1951-, Clark, Richard F., Kearney, Thomas E. (Seventh ed.). [NY]. ISBN 9780071839808. OCLC 1013928560.CS1 maint: others (link) - Critical Help
. Oropello, John M., Pastorez, Stephen M., Kvetan, Vladimir. [NY]. 2016-11-22. ISBN 9780071817264. OCLC 961480454 .CS1 maint: others (link) - Current Medical Diagnosis and Treatment 2020
. Papadakis, Maxine A., McPhee, Stephen J., Rabow, Michael W. (fifty-eighth ed.). NY. 2019-09-02. ISBN 9781260455281. OCLC 1109935506 .CS1 maint: others (link) - Harrison's Principles of Internal Medicine
. Jameson, J. Larry, Casper, Dennis L., Longo, Dan L. (Dan Louis), 1949-, Fauci, Anthony S., 1940-, Houser, Stephen L., Loscalzo, Joseph (20th ed.) . NY. 2018-08-13. ISBN 9781259644030. OCLC 1029074059 .CS1 maint: others (link) - Clinical anesthesiology by Morgan and Michael
. Butterworth, John F., IV, McKee, David K., Wasnik, John D., Morgan, J. Edward, Mikhail, Maged S., Morgan, J. Edward. (Sixth ed.). NY. 2018-08-21. ISBN 978-1259834424. OCLC 1039081701.CS1 maint: others (link) - Katsung, Bertram G. (2018-09-05). Katsung and Trevor Pharmacology: Exam and Board Review
. Kruidering-Hall, Marieke, Trevor, Anthony J. (Twelfth ed.). NY. ISBN 978-1259641022. OCLC 1052466341. - Levitsky, Michael G. (2007). Pulmonary physiology
(7th ed.). New York: McGraw-Hill Medical. ISBN 9780071437752. OCLC 75713147. - Field, Michael J.; Pollock, Carol A.; Harris, David S. (2010). The renal system: basic sciences and clinical settings
(2nd ed.). Edinburgh: Churchill Livingston/Elsevier. ISBN 9780702033711. OCLC 319855752. - Meert, K. L; Clark, J; Sarnaik, A. P (2007). "Metabolic acidosis as a major mechanism of respiratory distress in children with severe acute asthma." Pediatric resuscitation
.
8
(6): 519–23. DOI: 10.1097/01.PCC.0000288673.82916.9D. PMID 17906597. S2CID 27488853. - Costanzo, Linda S. (2017-03-15). Physiology
(6th ed.). Philadelphia, PA: Elsevier. ISBN 9780323511896. OCLC 965761862. - Kraut, Jeffrey A.; Madias, Nikolaos E. (4 September 2012). "Treatment of acute metabolic acidosis: a pathophysiological approach." Nature Reviews Nephrology
.
8
(10): 589–601. DOI: 10.1038/nrneph.2012.186. PMID 22945490. S2CID 34657707. - ^ a b
Kraut, Jeffrey A.;
Madias, Nikolaos E. (2017). "Adverse effects of metabolic acidosis in chronic kidney disease." Advances in the treatment of chronic kidney disease
.
24
(5): 289–297. DOI: 10.1053/j.ackd.2017.06.005. PMID 29031355. - Kato, Akihiko; Kido, Ryo; Onishi, Yoshihiro; Kurita, Noriaki; Fukagawa, Masafumi; Akizawa, Tadao; Fukuhara, Shunichi (2014). "The Association of Serum Bicarbonate with Bone Fractures in Hemodialysis Patients: A Study of Mineral and Bone Outcomes in Japanese Patients with CKD Stage 5D (MBD-5D)." Nephron Clinical Practice
.
128
(1–2): 79–87. DOI: 10.1159/000365089. ISSN 1660-2110. PMID 25378374. S2CID 20320396. - Lefebvre, A.; de Vernejoul, M.C.; Gueris, J.; Goldfarb, B.; Graulet AM; Moriyo, K. (1989). "Optimal correction of acidosis modifies the progression of dialysis osteodystrophy." Kidney International
.
36
(6):1112–1118. DOI: 10.1038/ki.1989.309. ISSN 0085-2538. PMID 2557481. - Hanna, Rami M.; Gobry, Lena; Wassef, Olivia; Rea, Connie M.; Kalantar-Zadeh, Kamyar (2020). "A practical approach to nutrition, protein-energy waste, sarcopenia and cachexia in patients with chronic kidney disease". Blood purification
.
49
(1–2): 202–211. DOI: 10.1159/000504240. ISSN 0253-5068. PMID 31851983. S2CID 209418220. - Foley, Robert N.; Wang, Changchun; Ishani, Arif; Collins, Allan J.; Murray, Anne M. (2007). "Kidney function and sarcopenia in the general population of the United States: NHANES III." American Journal of Nephrology
.
27
(3):279–286. DOI: 10.1159/000101827. ISSN 0250-8095. PMID 17440263. S2CID 2847009. - Shah, Samir N.; Abramowitz, Matthew; Hostetter, Thomas H.; Melamed, Michal L. (2009-08-01). "Serum bicarbonate levels and progression of kidney disease: a cohort study". American Journal of Kidney Diseases
.
54
(2):270–277. DOI: 10.1053/j.ajkd.2009.02.014. ISSN 1523-6838. PMC 4354889. PMID 19394734. - Good, Mirela; Yang, Wei; Chen, Jing; Drawz, Paul; Hamm, L. Lee; Horwitz, Edward; Hostetter, Thomas; Jaar, Bernard; Laura, Claudia M.; Nessel, Lisa; Ojo, Akinlolu (01/10/2013). "Association of serum bicarbonate with the risk of renal and cardiovascular outcomes in CKD: report from the Chronic Renal Insufficiency Cohort Study (CRIC)". American Journal of Kidney Diseases
.
62
(4):670–678. DOI: 10.1053/j.ajkd.2013.01.017. ISSN 0272-6386. PMC 3701754. PMID 23489677. - Menon, Vandana; Tigiwart, Hoshin; Vaughn, Nubia Smith; Beck, Gerald J.; Kusek, John W.; Collins, Allan J.; Green, Tom; Sarnak, Mark J. (2010-11-01). "Serum bicarbonate and long-term outcomes in CKD". American Journal of Kidney Diseases
.
56
(5):907–914. DOI: 10.1053/j.ajkd.2010.03.023. ISSN 0272-6386. PMID 20605301. - Raphael, Kalani L.; Wei, Guo; Baird, Bradley C.; Green, Tom; Beddhu, Srinivasan (02/01/2011). "Higher serum bicarbonate levels within the normal range are associated with better survival and improved kidney function in African Americans". Kidney International
.
79
(3):356–362. DOI: 10.1038/ki.2010.388. ISSN 0085-2538. PMC 5241271. PMID 20962743. - Jaber, Samir; Pogam, Catherine; Foutier, Emmanuel; Lefran, Jean-Yves; Lasocki, Sigismond; Lesko, Thomas; Pottecher, Julien; Demoule, Alexander; Ferrandier, Martina; Asehnoun, Karim; Dellamonica, Jean (07/07/2018). "Sodium bicarbonate therapy for patients with severe metabolic acidemia in the intensive care unit (BICAR-ICU): a phase 3, multicenter, open-label, randomized controlled trial" (PDF). Lancet
.
392
(10141):31–40. DOI: 10.1016/S0140-6736(18)31080-8. ISSN 0140-6736. PMID 29910040. S2CID 49276138. - Goraya, Nimrit; Wesson, Donald E. (2019). "Clinical evidence that treatment of metabolic acidosis slows the progression of chronic kidney disease". Current Opinion in Nephrology and Hypertension
.
28
(3):267–277. DOI: 10.1097/MNH.0000000000000491. ISSN 1062-4821. PMC 6467553. PMID 30681417. - Garneata, Liliana; Stancu, Alexandra; Dragomir, Diana; Stefan, Gabriel; Mircescu, Gabriel (07/01/2016). "Very low protein vegetarian diet supplemented with keto analogues and progression of CKD". Journal of the American Society of Nephrology
.
27
(7):2164–2176. DOI: 10.1681/ASN.2015040369. ISSN 1046-6673. PMC 4926970. PMID 26823552. - Chen, Wei; Abramowitz, Matthew K. (2019). "Advances in the treatment of chronic metabolic acidosis in chronic kidney disease". Current Opinion in Nephrology and Hypertension
.
28
(5): 409–416. DOI: 10.1097/MNH.0000000000000524. ISSN 1473-6543. PMC 6677263. PMID 31232712. - Wesson, Donald E.; Mathur, Vandana; Tangri, Navdeep; Stasov, Yuri; Purcell, Dawn; Lee, Elizabeth; Klaerner, Gerrit; Bushinsky, David A. (2019-04-06). "Veverimer versus placebo in patients with metabolic acidosis associated with chronic kidney disease: a multicenter, randomized, double-blind, controlled phase 3 trial". Lancet
.
393
(10179):1417–1427. DOI: 10.1016/S0140-6736(18)32562-5. ISSN 0140-6736. PMID 30857647. S2CID 72332908. - Wesson, Donald E.; Mathur, Vandana; Tangri, Navdeep; Stasov, Yuri; Purcell, Dawn; Lee, Elizabeth; Klaerner, Gerrit; Bushinsky, David A. (2019-08-03). "Long-term safety and efficacy of veverimer in patients with metabolic acidosis from chronic kidney disease: a multicenter, randomized, blinded, placebo-controlled, 40-week extension". Lancet
.
394
(10196):396–406. DOI: 10.1016/S0140-6736(19)31388-1. ISSN 0140-6736. PMID 31248662. S2CID 195339720.
Excretory acidosis
The main reason is the loss of bases (alkalies) by the body as a result of certain pathologies.
For example, with renal failure, hypoxia of kidney tissue or nephritis, a renal type of excretory acidosis develops. With an open wound of the intestine and diarrhea, intestinal excretory acidosis develops.
Inflammation in a particular organ and oxidative stress are cyclical processes, because an increase in inflammatory mediators and the circulation of immune system cells provokes an increasing formation of ROS by activating enzymes that generate radicals.
Mixed acidosis.
The same patient may exhibit signs of both gas and non-gas acidosis.
Examples of conditions in which mixed (combined) acidosis can develop: heart failure, brain injury, chronic broncho-obstructive pulmonary diseases, chronic gastroenteritis.
In any chronic disease, as a result of a constant, ongoing inflammatory process, the accumulation of markers of oxidative stress and the action of reactive oxygen species on tissue, blood acidification is observed.
Symptoms of acidosis
Symptoms of acidosis are sometimes difficult to distinguish from other pathological conditions.
The main symptoms of acidosis are:
- Short-term nausea, vomiting;
- General malaise;
- Increased heart rate
- Dyspnea;
- Cardiac arrhythmias;
- Increased blood pressure;
- Disorder of the functions of the central nervous system (drowsiness, confusion, dizziness, loss of consciousness, lethargy);
- Shock states
Oxidative stress is a precursor to acidosis.
As is known, free radicals can damage almost all cell structures: membrane proteins and lipids, DNA, thereby disrupting the vital functions of cells.
The accumulation of under-oxidized products in the cell leads to a disruption of the redox potential of cell membranes, which complicates the supply of substances necessary for functioning, as well as the removal of decay products.
As a result, the cytoplasm of the cells becomes acidified and intracellular metabolic acidosis develops.
Consequences and complications
Complications of metabolic acidosis can include:
- dehydration;
- decrease in circulating blood volume;
- increased blood clotting, accompanied by a risk of thrombosis;
- circulatory disorders (including such serious ones as myocardial infarction, infarction of parenchymal organs, peripheral thrombosis, stroke);
- arterial hypo- and hypertension;
- disturbance of brain functions;
- coma;
- death.
Diagnosis of acidosis
The following research methods are used to diagnose acidosis:
- Analysis of blood gas composition (for analysis, arterial blood is taken; analysis of venous blood will not accurately determine the pH level);
- Urine pH level analysis; Arterial blood analysis for serum electrolytes.
- Blood tests for basic metabolic parameters (blood gas composition) show not only the presence of acidosis, but also determine the type of acidosis (respiratory, metabolic).
What to do with ketoacidosis
What you can do at home:
- make correction insulin injections with a syringe pen
- when blood sugar drops, take fast carbohydrates with a joke
- drink more water
- measure blood sugar more often
- give yourself time to rest, come to your senses
- eliminate the cause of DKA (replace the infusion set, change insulin to a more recent one, etc.)
- Monitor ketone levels for at least 24 hours after the incident
If you have symptoms of a hypersmolar condition / if, despite the measures taken, the condition does not improve, call 112!
Treatment of acid-base imbalance
To treat acidosis, it is necessary to determine what type of acidosis and set a goal to reduce the symptoms of this type. It is necessary to influence the root cause of acidosis, and not just alkalize the body with alkaline products, because alkalizing the body will only bring short-term benefits and will lead to acidosis again.
Respiratory acidosis
The main goal: to reduce the degree of respiratory failure in the first place, as well as symptomatic treatment aimed at eliminating unpleasant sensations: headache, overexcitement, severe cardiac dysfunction.
Non-gas acidosis
The main goal: eliminate excess acids from the body, restore the normal content of bicarbonates.
The first step is to eliminate the disease or pathological condition (for example, oxidative stress that causes inflammation) that causes acidosis.
Symptomatic treatment is aimed at eliminating heart rhythm disorders, gastrointestinal tract functions, and disorders of neuromuscular tone.
Acid-base balance is an important parameter that is maintained in human blood within certain limits. This is necessary for the normal functioning of various body systems, the occurrence of biochemical reactions, and the optimal functioning of enzymes.
Acids are substances that can give off hydrogen ions, and bases (alkalis) are substances that attach these ions. The acidity and alkalinity of solutions is assessed on a pH scale from 0 (solutions of strong acids) to 14 (solutions of strong alkalis). On the pH scale, neutral acidity is 7.
Normal blood acidity is 7.35 – 7.45 on the pH scale. A shift of this indicator below 7.35 indicates acidosis (a shift in the acid-base balance of the blood towards increasing acidity). When the pH deviates above 7.45, alkalosis occurs (excess of substances with alkali properties in the blood).
During the process of metabolism in the body, products are formed in large quantities that can cause a change in this parameter. The main role in the regulation of acid-base balance belongs to the lungs, kidneys and blood buffer systems.
During breathing, carbon dioxide is released through the lungs, which is formed during the metabolic process in the body. Carbon dioxide, when combined with water, forms carbon dioxide, therefore, if there is an excess of it in the blood, acidosis develops, and if the concentration of carbon dioxide is insufficient, alkalosis occurs.
The kidneys remove excess acids and alkalis from the body with urine. At the same time, these organs, within certain limits, can regulate the amount of acids and bases released and absorbed back, due to which the pH level in the blood is regulated.
Blood buffer systems are solutions of weak acids and alkalis, which combine with excess amounts of acids or bases (depending on the presence of acidosis or alkalosis) to neutralize them, thereby equalizing the pH level.
The cause of acidosis and alkalosis in most cases is the severe course of the underlying disease, in which the resulting changes in blood pH exceed the capabilities of the mechanisms regulating this parameter.
Synonyms Russian
Disorders of the acid-base balance of the blood, disturbances of acid-base homeostasis.
English synonyms
Acid-Base Disorders, Acid-Base Homeostasis.
Symptoms
Manifestations of acidosis and alkalosis are often masked by manifestations of the underlying disease, which caused a change in the acid-base balance of the blood.
Acidosis may have the following symptoms:
- nausea, vomiting
- increased breathing rate
- headache
- disturbance of consciousness (up to coma)
- drop in blood pressure (in severe forms of acidosis)
- heart rhythm disturbances.
Manifestations of alkalosis may include:
- headache
- dizziness
- depression of consciousness (up to coma)
- cramps in various muscle groups
- heart rhythm disturbances
General information about the disease
The acid-base balance in the blood is a vital parameter, the normal values of which are 7.35 - 7.45 on the pH scale.
A pH deviation below 7.35 indicates acidosis. When the pH shifts above 7.45, alkalosis occurs.
Depending on the causes of development, acidosis and alkalosis are divided into metabolic (metabolic) and respiratory (breathing).
Respiratory acidosis develops as a result of the accumulation of large amounts of carbon dioxide in the blood, which combines with water to form carbon dioxide. This causes an increase in blood acidity. This condition can develop with breathing disorders that cause decreased pulmonary ventilation.
This may be the result of lung diseases (for example, bronchial asthma), damage to the nervous system (for example, with brain injuries), diseases, muscles and nerves that lead to loss of the ability to make effective breathing movements (for example, with amyotrophic lateral sclerosis).
The opposite condition is respiratory alkalosis, which occurs when the lungs remove excess carbon dioxide from the body. The mechanism for the development of this type of alkalosis is based on an increase in the rhythm and depth of breathing.
Such breathing disorders can occur in the presence of pathology from various organs and systems (for example, injuries, brain tumors, lung diseases, cardiovascular failure).
Metabolic acidosis can develop for the following reasons:
- increased production of acids in the body. An increase in acid production in the body can be observed in conditions accompanied by metabolic disorders. For example, in diabetes mellitus, the use of glucose by cells is impaired due to a lack of the hormone insulin.
At the same time, the body begins to produce energy not from glucose, but from fats - an alternative way to obtain energy. The breakdown of fats in the liver is accompanied by the formation of large quantities of ketone acids, which leads to acidosis.
- impaired renal function. The kidneys play an important role in regulating the acid-base balance in the blood. In case of kidney diseases that lead to disruption of their functions, the processes of acid secretion and absorption of substances with an alkaline reaction may be disrupted, which can cause acidosis.
- loss of large quantities of alkalis with digestive juices. This condition can be observed with severe diarrhea, surgical interventions on the intestines.
- poisoning with poisons and toxic substances. The breakdown of these substances in the body can occur with the formation of large amounts of acids, which can cause acidosis.
The main causes of metabolic alkalosis are the following:
- loss of large amounts of acidic gastric contents. It can be observed with profuse vomiting, aspiration of stomach contents using a special probe.
- use of diuretics
- increased excretion of hydrogen ions by the kidneys. Such processes can be observed with an excess of the adrenal hormone aldosterone. Aldosterone is involved in the regulation of water and electrolyte balance in the body. An increase in its level can occur both in diseases of the adrenal glands and in pathologies of other organs (for example, heart failure).
Thus, the development of acidosis or alkalosis is often associated with the occurrence of pathological processes in which the resulting changes in acid-base balance exceed the compensatory capabilities of the body. In this case, an important role in treatment is played by the normalization of the patient’s condition for the underlying disease that caused the deviation in blood pH.
Who is at risk?
The risk group for developing acid-base balance disorders in the blood includes:
- persons suffering from lung diseases (for example, bronchial asthma)
- persons with kidney disease with impaired kidney function
- persons suffering from diabetes mellitus
- persons with damage to the nervous system (for example, brain injuries, strokes)
- persons who have suffered large losses of gastrointestinal contents (for example, with excessive vomiting, frequent loose stools)
- persons taking certain medications (eg, diuretics, aspirin)
- persons who abuse alcohol.
Diagnostics
Laboratory research methods play an important role in diagnosis, which allows one to establish the blood pH level, its gas composition, parameters of water-electrolyte metabolism and other vital indicators, monitoring and correction of which are necessary for these conditions.
Laboratory research:
- Determination of blood pH, blood gas composition. Determination of these parameters can be carried out using special devices - gas analyzers. The material for the study is arterial blood.
- General blood analysis. This analysis allows you to evaluate the main characteristics of blood composition: the number of red blood cells, hemoglobin, leukocytes, platelets. This study is not specific for diagnosing acidosis or alkalosis, but is necessary to identify the causes of changes in blood pH.
- General urine analysis with microscopy. This analysis shows the basic physicochemical properties of urine, its pH level, and the presence of pathological and physiological metabolic products.
- Glucose in blood plasma. Glucose is the main source of energy in the human body. An increase in blood glucose levels is observed in diabetes mellitus. Metabolic disorders that occur with this disease can lead to the development of acidosis.
- Potassium, sodium, chlorine in serum. Potassium, sodium, and chlorine are the main electrolytes in the human body that perform many functions. Among them are participation in the transport of substances into the cell and the removal of metabolic products from it, maintaining water and acid-base balance in the body.
- Alanine aminotransferase (ALT). Alanine aminotransferase is an enzyme found in many cells of the body. Most of it is concentrated in the liver. When the liver is damaged, the level of this enzyme in the blood increases. Impaired liver function can lead to changes in the acid-base balance in the blood.
- Creatinine and urea in blood serum. Creatinine and urea are the end products of protein metabolism in the human body. They are excreted from the body by the kidneys. If kidney function is impaired, an increase in these indicators may be observed. Kidney damage can lead to changes in the acid-base balance in the body.
Depending on the specific clinical situation, other laboratory tests may be required to identify the causes of acidosis or alkalosis (for example, determining the level of ketone bodies in the blood and urine, the concentration of lactate in the blood, etc.).
Research:
- Radiography. Chest x-rays can detect pathological changes in the lungs (for example, pneumonia) that result in changes in the rhythm and depth of breathing.
- Ultrasound examination (ultrasound). The method is based on the properties of ultrasound. Using ultrasound, you can visualize internal organs, identify changes in their structure, the presence of space-occupying formations (for example, cysts, tumors), which may be necessary to determine the causes of disturbances in the acid-base balance in the blood.
- Computed tomography (CT). The method allows you to obtain layer-by-layer
highly informative images of internal organs. This is of great importance for identifying the disease that caused acidosis or alkalosis (for example, respiratory failure resulting from cerebral hemorrhage).
Treatment
Treatment of disorders of the acid-base balance in the blood is aimed at treating the underlying disease that led to the development of acidosis or alkalosis. To normalize the pH level, intravenous administration of solutions that neutralize acids (for acidosis) or alkalis (for alkalosis) can be carried out.
Treatment of respiratory acidosis is aimed at restoring the rhythm and depth of breathing with the possible transfer of the patient to artificial ventilation (breathing using a special apparatus in cases of ineffective lung function).
For respiratory alkalosis, inhalation of air mixtures containing carbon dioxide can be used.
Prevention
There is no specific prevention of changes in the acid-base balance in the blood. Patients suffering from diseases that can cause changes in blood pH (for example, diabetes) should strictly follow the recommendations of their doctor and undergo regular examinations and treatment.
Recommended tests
- Blood pH determination
- Determination of blood gas composition
- General blood analysis
- General urine analysis with microscopy
- Blood plasma glucose
- Potassium, sodium, chlorine in serum
- Alanine aminotransferase (ALT)
- Serum creatinine
- Urea in serum