Composition and release form
The drug is sold in pharmacies in the form of small tablets for oral administration. Externally, they are white in color with a soluble thin shell. Packaged in blisters of 14 pieces and secondary cardboard packaging. One pack contains 1-2 cells of 14-28 tablets and includes instructions for use.
The active component of the drug is moxonidine. Its amount in 1 tablet is 200 mcg.
Additional substances: castor oil, cellulose, magnesium stearate, Tween 80, Klucel and Aerosil.
Pharmacokinetics
Van suctions:
After oral administration, moxonidine is rapidly and almost completely absorbed into the
upper parts of the gastrointestinal tract. Absolute bioavailability is approximately 88%. Time to reach maximum concentration -; about 1 hour. Food intake does not affect the pharmacokinetics of the drug.
Distribution
The connection with blood plasma proteins is 7.2%.
Metabol Meas.
The main metabolite is; dehydrogenated moxonidine. Pharmacodynamic activity of dehydrogenated moxonidine -; about 10% compared to moxonidine.
Output
The half-life (T1/2) of moxonidine and metabolite is 2.5 and 5 hours, respectively. Within 24 hours, over 90% of moxonidine is excreted by the kidneys (about 78
% unchanged and 13% in the form of dehydri-romoxonidine, other metabolites in the urine do not exceed 8% of the dose taken). Less than 1% of the dose is excreted through the intestines.
Pharmacokinetics in patients with arterial hypertension:
Compared with healthy volunteers, patients with arterial hypertension show no changes in the pharmacokinetics of moxonidine.
Farmak okin etika in old age
Clinically insignificant changes in the pharmacokinetic parameters of moxonidine were noted in elderly patients, probably due to a decrease in the intensity of its metabolism and/or slightly higher bioavailability.
Farmak okinetics in children
Moxonidine is not recommended for use in patients under 18 years of age, and therefore pharmacokinetic studies have not been conducted in this group.
Pharmacokinetics for renal failure
Moxonidine excretion is significantly correlated with creatinine clearance (CC). In patients with moderate renal failure (creatinine clearance in the range of 30-60 ml/min), steady-state plasma concentrations and final T1/2 are approximately 2 and l.5 times higher than in patients with normal renal function (creatinine clearance more than 90 ml/min). min.).
In patients with severe renal failure (creatinine clearance less than 30 ml/min.), steady-state plasma concentrations and final T1/2 are 3 times higher than in patients with normal renal function. The administration of multiple doses of moxonidine leads to predictable accumulation in the body of patients with moderate and severe renal failure. In patients with end-stage renal failure (creatinine clearance less than 10 ml/min) on hemodialysis, steady-state plasma concentrations and final T1/2 are 6 and 4 times higher, respectively, than in patients with normal renal function. In all groups, the maximum concentration of moxonidine in blood plasma was 1.5 - 2 times higher. U
For patients with impaired renal function, the dosage should be adjusted individually. Moxonidine is excreted to a small extent during hemodialysis.
Indications for use
Arterial hypertension.
Contraindications
- hypersensitivity to the active substance and other components of the drug;
— sick sinus syndrome;
— severe bradycardia (resting heart rate less than 50 beats/min);
— atrioventricular block II and III degrees;
- severe heart rhythm disturbances;
— acute and chronic heart failure (III-IV functional class according to the NYHA classification);
- simultaneous use with tricyclic antidepressants (see section
“Interaction with other drugs”);
- severe renal failure (creatinine clearance less than 30 ml/min);
- hemodialysis;
- lactation period;
- hereditary lactose intolerance, lactase deficiency or glucose-galactose malabsorption;
— age over 75 years;
- age under 18 years (due to the lack of data on safety and effectiveness).
Particular caution should be exercised when using moxonidine in patients with
atrioventricular block of the first degree (risk of developing bradycardia); severe coronary artery disease, severe coronary heart disease or unstable angina (insufficient experience), chronic heart failure, severe liver failure, with impaired renal function (creatinine clearance more than 30 ml/min).
Pregnancy
There are no clinical data on the use of Moxonidine in pregnant women.
In animal studies, the embryotoxic effect of the drug was established. Moxonidine should be prescribed to pregnant women only after a careful assessment of the risk-benefit ratio, when the benefit to the mother outweighs the potential risk to the fetus.
Lactation period
Moxonidine passes into breast milk and should therefore not be given during breastfeeding. If it is necessary to use Moxonidine during lactation, breastfeeding should be stopped.
Directions for use and doses
Inside, regardless of food intake. In most cases, the initial dose of the drug
Moxonidine is 0.2 mg per day. The maximum single dose is 0.4 mg. The maximum daily dose, which should be divided into 2 doses, is 0.6 mg. Individual adjustment of the daily dose is necessary depending on the patient’s tolerance to the therapy. No dose adjustment is required for patients with hepatic impairment. The starting dose for patients with moderate or severe renal impairment is 0.2 mg/day. If necessary and if well tolerated, the daily dose can be increased to a maximum of 0.4 mg.
Side effect
The frequency of side effects listed below was determined accordingly
the following: very often (>1/10); often (>1/100, <1/10); uncommon (>1/1000, <1/100); including individual messages.
From the central nervous system:
Common: headache*, dizziness (vertigo), drowsiness. Uncommon: fainting*.
From the cardiovascular system:
Uncommon: marked decrease in blood pressure, orthostatic hypotension*, bradycardia.
From the gastrointestinal tract:
Very common: dry mouth. Common: diarrhea, nausea, vomiting, dyspepsia.
From the skin and subcutaneous tissues: Often: skin rash, itching.
Uncommon: angioedema.
Mental disorders:
Common: insomnia. Uncommon: nervousness.
Hearing and labyrinthine disorders: Uncommon: ringing in the ears.
Musculoskeletal and connective tissue disorders: Common: back pain.
Uncommon: neck pain.
General disorders and disorders at the injection site:
Often: asthenia.
Uncommon: peripheral edema.
(* - frequency comparable to placebo).
Overdose
There have been several reports of non-fatal overdoses when
Doses up to 19.6 mg were used simultaneously.
Symptoms:
headache, sedation, marked decrease in blood pressure, dizziness, asthenia, bradycardia, dry oral mucosa, vomiting, fatigue, pain in the epigastric region, respiratory depression and impaired consciousness. In addition, short-term increases in blood pressure, tachycardia and hyperglycemia are also possible, as shown in several high-dose animal studies.
Treatment
There is no specific antidote. In the case of a pronounced decrease in blood pressure, it may be necessary to restore the volume of circulating blood by introducing fluid and dopamine (injection).
Bradycardia can be stopped with atropine (injection).
In severe cases of overdose, it is recommended to carefully monitor disturbances of consciousness and avoid respiratory depression.
Alpha-adrenergic antagonists may reduce or eliminate the paradoxical hypertensive effects of moxonidine overdose.
Moxonidine is excreted to a small extent during hemodialysis.
Interaction with other drugs
The combined use of moxonidine with other antihypertensive drugs leads to an additive effect. Tricyclic antidepressants may reduce the effectiveness of centrally acting antihypertensive drugs, and therefore their use together with moxonidine is not recommended.
Moxonidine may enhance the effect of tricyclic antidepressants, tranquilizers, ethanol, sedatives and hypnotics.
Moxonidine may moderately improve impaired cognitive function in patients receiving lorazepam.
Moxonidine may enhance the sedative effect of benzodiazepine derivatives when administered simultaneously.
Moxonidine is released by tubular secretion. Therefore, its interaction with other drugs released by tubular secretion is not excluded. Beta-blockers increase bradycardia and the severity of negative ino- and dromotropic effects.
special instructions
If it is necessary to cancel beta-blockers and the drug Moxonidine taken simultaneously, first cancel the beta-blockers and only after a few days Moxonidine.
There is currently no evidence that stopping Moxonidine leads to an increase in blood pressure. However, it is not recommended to stop taking Moxonidine suddenly; instead, you should gradually reduce the dose of the drug over two weeks.
Avoid drinking alcohol during treatment.
During treatment, regular monitoring of heart rate and electrocardiography is necessary.
Impact on the ability to drive vehicles and operate machinery
The effect of Moxonidine on the ability to drive vehicles or operate machinery has not been studied. However, taking into account the possibility of dizziness and drowsiness, patients should be careful when engaging in potentially hazardous activities that require increased attention, such as driving a vehicle or operating equipment that requires increased concentration.
Release form
Film-coated tablets, 0.2 mg, 0.3 mg and 0.4 mg
10, 14 or 30 tablets per blister pack.
60 tablets in polymer jars or polymer bottles.
Each jar, bottle, 3 blister packs of 10 tablets, 1 or 2 blister packs of 14 tablets each, or 1 or 2 blister packs of 30 tablets, together with instructions for use, are placed in a cardboard box.
Best before date
3 years. Do not use after the expiration date stated on the packaging.
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 C.
Keep out of the reach of children.
Vacation conditions
On prescription
pharmachologic effect
The drug belongs to the group of antihypertensive drugs. It is a direct agonist of imidazoline receptors. When used once, it helps to simultaneously restore the level of diastolic and systolic pressure. At the same time, it does not change the activity of the heart and does not affect the heart rate.
The drug is highly digestible, regardless of the time of meal. The biological availability of the drug is at least 88%. The highest concentration of the drug in the red channel is observed after 30 minutes or a maximum of 3 hours.
The role of moxonidine in the treatment of arterial hypertension
The article presents data on the antihypertensive effectiveness of the imidazoline receptor agonist moxonidine and the possibilities of its use as part of combination therapy for arterial hypertension. The mechanisms of the antihypertensive effect of the drug, its effect on carbohydrate metabolism, and tissue sensitivity to insulin are considered. Moxonidine has an organoprotective effect, and due to its ability to reduce insulin resistance, it is recommended for the treatment of arterial hypertension in patients with metabolic syndrome, diabetes mellitus, and obesity.
Arterial hypertension (AH) is a serious health problem and is included in the list of socially significant diseases, since its prevalence is high (up to 40% among the adult population of Russia) and contributes to an increased risk of cardiovascular complications. The incidence of hypertension increases with age and reaches 50–60% in people over 60 years of age [1].
According to the latest national recommendations and the recommendations of the European Society of Cardiology (ESC), the main goal of hypertension treatment is to minimize the risk of developing cardiovascular complications and thereby cardiovascular death [2, 3].
To achieve this goal, it is necessary to achieve the target level of blood pressure (BP), reduce the severity of the patient’s modifiable risk factors or eliminate them (smoking, dyslipidemia, obesity, etc.), achieve regression of identified target organ damage and, finally, effectively treat associated diseases, primarily coronary heart disease (CHD) and diabetes mellitus (DM). The presence of coronary artery disease and type 2 diabetes in patients with hypertension means a high or very high cardiovascular risk. Therefore, the antihypertensive therapy chosen for these patients should, at a minimum, not worsen the course of concomitant diseases.
Previously, an increased risk of myocardial infarction (for rauwolfia preparations) and a critical deterioration of peripheral circulation in patients with diabetes with macroangiopathies in response to the use of non-selective beta-blockers were noted. In addition, some antihypertensive drugs that effectively lower blood pressure can have an adverse effect on carbohydrate and lipid metabolism (non-selective beta-blockers, thiazide diuretics in high doses).
For more than ten years, a program to combat hypertension has been implemented in Russia, and the reduction in cardiovascular mortality achieved in recent years is associated with successes in the treatment of hypertension. However, the frequency of achieving the target blood pressure level (
For the treatment of hypertension, modern recommendations propose five main classes of drugs: angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, slow calcium channel blockers, and diuretics. Additional antihypertensive drugs include selective I1-imidazoline receptor agonists, in particular the drug moxonidine, and alpha-blockers (doxazosin).
Since activation of the sympathetic nervous system (SNS) plays an important role in the pathogenesis of hypertension, for the treatment of hypertension it is advisable to prescribe drugs that can reduce the activity of the SNS, primarily beta-blockers. However, in some patients their prescription is impossible due to the presence of absolute or relative contraindications. In this situation, second-generation centrally acting drugs, such as selective I1-imidazoline receptor agonists (moxonidine, rilmenidine), can be used.
Mechanism of action of moxonidine
Moxonidine is an imidazoline receptor agonist. There are three types of imidazoline receptors [4–6]. Type 1 imidazoline receptors are localized in the central nervous system (in the nuclei of the reticular formation, rostral ventrolateral region of the medulla oblongata). Their activation leads to a decrease in the activity of the sympathoadrenal system, the level of norepinephrine and, consequently, the level of blood pressure due to a decrease in peripheral vascular resistance while simultaneously reducing the heart rate. Type 2 imidazoline receptors are located in the sympathetic nerve endings (they regulate the release of norepinephrine and adrenaline) and the kidneys (when stimulated, the level of plasma renin decreases and a moderate natriuretic effect is provided). Type 3 imidazoline receptors are found in the pancreas (they regulate insulin secretion by the beta cells of the islets of Langerhans).
An increase in the level of atrial natriuretic peptide may also contribute to the antihypertensive effect of moxonidine [7].
Pharmacokinetics
When taken orally, 90% of moxonidine is absorbed from the gastrointestinal tract, the maximum concentration is reached after 60 minutes [8]. The half-life of moxonidine and its metabolites is 2.5 and 5 hours, respectively. The antihypertensive effect continues throughout the day due to strong binding to I1-imidazoline receptors in the medulla oblongata. Moxonidine is excreted primarily in the urine (more than 90% of the drug is excreted within 24 hours) by glomerular filtration and tubular secretion and does not accumulate with long-term use, including in patients with moderate renal failure. In patients with end-stage renal failure (creatinine clearance less than 10 mg/min), the concentration of moxonidine in the blood plasma may be several times higher compared to patients with normal renal function.
Application in clinical practice
The indication for the use of moxonidine is hypertension. The antihypertensive effect after taking 0.2–0.4 mg of the drug begins after 30 minutes, reaches a maximum after 2–5 hours and lasts up to 24 hours. Moxonidine does not cause an excessive decrease in blood pressure at night, but at the same time effectively prevents the rise in blood pressure in the early morning hours.
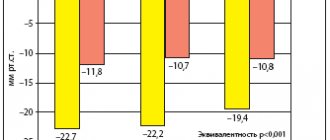
When treated with moxinidine, there is a decrease in systolic blood pressure by 20–30 mmHg. Art., diastolic - by 10–20 mm Hg. Art. The greatest antihypertensive effect is achieved after three weeks of therapy, and its evasion is not observed [9]. In contrast to first-generation centrally acting drugs (in particular, clonidine), when moxonidine is stopped, no hypertension syndrome is observed [10] - blood pressure gradually returns to its original level.
The antihypertensive effectiveness of moxonidine is comparable to that of diuretics, beta-blockers, calcium antagonists and ACE inhibitors [11–15]. The ratio of residual to maximum reduction in blood pressure for moxonidine is 70% [15]. This is a very important indicator that characterizes the duration of the antihypertensive effect and, therefore, the possibility of prescribing the drug once a day.
The work of W. Scwarz et al. demonstrated that therapy with moxonidine at a dose of 0.2 mg/day in patients with hypertension of the first and second degree helps to normalize blood pressure in 62% of cases. In another 36% of patients, normalization of blood pressure was achieved by increasing the dose to 0.4 mg/day. The maximum reduction in blood pressure is observed in the third week of therapy, the resulting effect persists throughout the year of observation [9].
In elderly patients with hypertension, moxonidine leads to a mild, gradual decrease in blood pressure, and is well tolerated [16]. It is important that no significant differences in the effectiveness and frequency of side effects of therapy were recorded in older and younger patients.
Moxonidine can also be used as part of combination antihypertensive therapy. The TOPIC study, conducted in the UK, examined the effectiveness of moxonidine both as monotherapy and as combination therapy in 566 patients with hypertension [17]. With monotherapy with moxonidine at a dose of 0.2–0.4 mg/day, blood pressure control was achieved in 52% of patients. In combination therapy with hydrochlorothiazide (25 mg/day), amlodipine (5 mg/day) or enalapril, the combination with the second drug was most effective.
J. Abellan et al. moxonidine was prescribed at a dose of 0.4 mg to 112 patients with hypertension and obesity in whom previous antihypertensive therapy was ineffective. After six months, there was a decrease in systolic blood pressure by an average of 23 mm Hg. Art., diastolic - by 12.9 mm Hg. Art. At the same time, 63% of study participants achieved the target level of systolic blood pressure, 86% – diastolic blood pressure [18].
Use for uncomplicated hypertensive crises
Since, when moxonidine is prescribed for hypertension, a higher initial level of blood pressure is associated with a stronger decrease in blood pressure, the possibility of using the drug to relieve hypertensive crises has been actively studied [19, 20]. With sublingual administration of moxonidine at a dose of 0.4 mg, a significant decrease in blood pressure was observed in 90% of patients [19]. A significant decrease in systolic and diastolic blood pressure after a single dose of moxonidine was recorded after 20 minutes and reached a maximum after 1.5 hours. According to the results of the AVES study, moxonidine with a single dose of 0.4 mg significantly reduces blood pressure levels after 30 minutes [21].
It is important that after taking moxonidine, blood pressure decreases gradually (no more than 25% of the initial level), which is an indispensable condition for the safe treatment of uncomplicated hypertensive crises. In this case, the effect lasts for 12 hours (unlike captopril, the duration of action of which does not exceed 6 hours) [22, 14].
Organoprotective effect
Moxonidine may promote regression of left ventricular hypertrophy. This effect was established when patients with hypertension were treated with moxonidine for six months, while the ejection fraction did not change significantly [23]. The reduction in left ventricular hypertrophy has been confirmed in experimental studies. The mechanism of action of moxonidine on the myocardium can be explained by the activation of imidazoline receptors localized in the heart, regulation of apoptosis processes and DNA stabilization [24].
In a group of patients with essential hypertension and microalbuminuria (n = 56), the effectiveness of moxonidine monotherapy was studied. Patients received the drug at a dose of 0.3–0.4 mg/day for six months. Moxonidine contributed to a significant decrease in blood pressure, a decrease in the severity of albuminuria, the concentration of plasma thrombomodulin and plasminogen activator inhibitor. The authors of the study associated the dynamics of the levels of these markers with the normalization of the endothelium during treatment [25].
The clinical and pharmacoeconomic efficacy of moxonidine and nitrendipine were compared in a prospective randomized trial. It included patients with hypertension who suffered from chronic renal failure. Over three years of observation, terminal chronic renal failure developed in 38% of cases in those receiving nitrendipine, and in 7% in those receiving moxonidine. The total cost of treatment with moxonidine was four times lower than the cost of treatment with nitrendipine [26].
The renoprotective properties of moxonidine were confirmed by J. Radermacher et al. [27]. In 601 patients undergoing renal allotransplantation, treatment with moxonidine resulted in a 70% reduction in the risk of allograft failure.
It has been established that the use of moxonidine can also reduce the frequency and duration of paroxysms of atrial fibrillation. Thus, 56 patients with paroxysmal atrial fibrillation received moxonidine or placebo for six weeks (a crossover design was used) under the control of 48-hour electrocardiography monitoring. During treatment with moxonidine, a significant decrease in diastolic blood pressure and a decrease in the average duration of episodes of atrial fibrillation per day from 28 to 16 minutes were noted. The authors suggested that the antiarrhythmic effect of moxonidine is associated with its sympatholytic activity [28].
Metabolic effects
Insulin resistance and hyperinsulinemia are the leading links in the pathogenesis of metabolic syndrome. The ability of moxonidine to influence tissue sensitivity to insulin has been studied in several studies [29]. Thus, G. Derosa et al. compared the effectiveness of moxonidine as monotherapy and in combination with irbesartan in 99 patients with hypertension and diabetes. For three months, all patients received moxonidine at a dose of 0.2 mg, then for some of them the dose of the drug was doubled, for others, irbesartan was added at a dose of 150 mg/day. In both groups, a significant decrease in blood pressure was recorded, however, only those taking moxonidine at a dose of 0.4 mg/day found a decrease in blood glucose levels, glycated hemoglobin, an improvement in the insulin sensitivity index, as well as a significant increase in high-density lipoprotein cholesterol levels [30].
Moxonidine modulates all three types of imidazoline receptors, thus exerting a complex effect on blood pressure levels and the metabolic profile.
The effect of moxonidine on metabolic status was demonstrated in the ALMAZ study. It was shown that tissue sensitivity to insulin increased equally with the administration of moxonidine and with the administration of metformin [31, 32]. Moxonidine therapy was accompanied by an improvement in carbohydrate and lipid metabolism parameters, a decrease in leptin levels, an improvement in endothelial function, and a decrease in body weight.
The effectiveness and safety of moxonidine in patients with hypertension and metabolic syndrome were assessed in a large multicenter international study, MERSY. Russia also took part in it (272 patients with hypertension and metabolic syndrome, including postmenopausal women). Almost half of the study participants, after adding moxonidine to therapy, had a target blood pressure level, and the effectiveness of antihypertensive therapy was higher in women with preserved menstrual function than in postmenopausal women (the target blood pressure level was achieved in 73 and 41% of cases, respectively). All patients showed a significant improvement in parameters characterizing carbohydrate and lipid metabolism. Particularly impressive was the reduction in triglyceride levels – by 24% [33].
The effect of moxonidine on insulin resistance is associated with an effect on sympathetic activity, which is accompanied by a decrease in fat hydrolysis, fatty acid content, the proportion of insulin-resistant (type IIB) fibers in skeletal muscles, and acceleration of glucose metabolism in tissues. It is the increase in tissue sensitivity to insulin that can explain the significant decrease in body weight in patients with metabolic syndrome who participated in both the MERSY study and the ALMAZ study.
The use of moxonidine promotes the activation of lipolysis of adipose tissue and weight loss. This was shown in the large open-label CAMUS study. 4005 patients with hypertension who also suffered from obesity or metabolic syndrome received moxonidine as monotherapy and as part of combination therapy. In addition to the hypotensive effect, moxonidine significantly reduced body weight. Moreover, the degree of its reduction depended on the initial body mass index. Over eight weeks of observation, during therapy with moxonidine, the body weight of patients in the group decreased by 1.4 kg on average, while in patients with third-degree obesity it decreased by 4 kg [34]. Based on the data obtained, the Russian Ministry of Health recommended moxonidine for the treatment of hypertension in this category of patients [35].
Moxonidine and chronic obstructive pulmonary disease
Moxonidine effectively reduces blood pressure in patients with chronic obstructive pulmonary disease (COPD), who often have problems when prescribed beta-blockers and ACE inhibitors. The former worsen bronchial obstruction, the latter provoke coughing and increase bronchial obstruction. Thus, during therapy with moxonidine in 40 patients suffering from hypertension and COPD, systolic and diastolic blood pressure decreased by 15.4 and 17.4%, respectively, while most of the patients achieved the target blood pressure level. In addition, hemodynamics in the systemic and pulmonary circulation improved [36].
Safety and Tolerability
Moxonidine, compared to first-generation centrally acting drugs (reserpine, clonidine, guanfacine, alpha-methyldopa), has a significantly lower affinity for alpha-2 adrenergic receptors in the brainstem, and therefore side effects such as dryness develop much less frequently with its use in the mouth and sedation. However, their severity decreases after a few weeks.
The safety and tolerability of moxonidine were analyzed based on the results of 74 clinical studies (370 thousand patient-years). The most common (more than 2% of patients) side effects of moxonidine are dry mouth, headache and weakness. The incidence of other side effects did not exceed 1%. The drug was discontinued due to the development of side effects in less than 4% of cases [37].
Conclusion
The selective inhibitor of I1-imidazoline receptors moxonidine can be considered as a universal antihypertensive drug, effective both for long-term treatment of hypertension and for the relief of uncomplicated hypertensive crises.
Moxonidine has an organoprotective effect, is easy to use (once a day in the morning), and is well tolerated, including in elderly patients.
The positive effect of moxonidine on carbohydrate and lipid metabolism makes it the drug of choice in patients suffering from diabetes and metabolic syndrome.
The drug can be used as monotherapy, as well as in combination with ACE inhibitors, ARBs, calcium antagonists, and diuretics.
Contraindications
It is prohibited to take the medicine for the following conditions:
- Individual intolerance to the active or auxiliary components.
- Acute form of bradycardia.
- Severe arrhythmia.
- Glaucoma.
- Mental disorders.
- Cardiac failure.
- Previous Quincke's edema.
- The period of bearing a child and breastfeeding.
- Children under 18 years of age.
- Disturbance of the liver and kidneys.
- Angina pectoris.
- Lactose deficiency or intolerance.
- Various pathologies of the circulatory system.
Side effects
As a result of treatment with Moxonidine, patients may experience the following adverse reactions:
- High fatigue, malaise.
- Migraine.
- Sleep disorder - insomnia or drowsiness.
- Dizziness, headache.
- Dyspeptic manifestations - dry mouth, discomfort in the epigastrium, nausea, vomiting, abnormal stool.
- Increased nervousness and irritability.
- Swelling of peripheral tissues.
- A sharp decrease in pulse and blood pressure.
- Allergic signs are skin rash and severe itching.
Drug overdose
If medical recommendations are not followed and the maximum volume of the drug is exceeded, an overdose may develop. The condition is determined by severe symptoms:
- Intense headache and dizziness.
- Nausea followed by vomiting.
- Painful sensations in the stomach area.
- Weakness and severe malaise.
- Extensive reduction in pressure.
- Dryness in the mouth.
If the above symptoms occur, you should definitely consult a doctor. Treatment involves relief of symptoms. Prescribed medication, gastric lavage, parenteral administration of saline. To suppress the symptoms of bradycardia, the use of Atropine is indicated.
special instructions
The use of an antihypertensive drug for therapeutic purposes requires constant monitoring of blood pressure, heart function and heart rate.
During the course of treatment, you should completely avoid alcoholic beverages. It is recommended not to drive vehicles or engage in activities that require concentration, memory and high mental activity.
You need to stop taking the drug gradually, gradually reducing the dose until complete withdrawal.
If the use of Moxonidine was combined with beta-blockers and it is necessary to discontinue both drugs, the latter are removed first. After some time, the antihypertensive drug is discontinued.
Pharmacodynamics
Moxonidine is an antihypertensive drug with a central mechanism of action. In the brainstem structures (rostral layer of the lateral ventricles), moxonidine selectively stimulates imidazoline-sensitive receptors that take part in the tonic and reflex regulation of the sympathetic nervous system. Stimulation of imidazoline receptors reduces peripheral sympathetic activity and blood pressure (BP).
Moxonidine differs from other sympatholytic antihypertensive drugs in its lower affinity for α2-adrenergic receptors, which explains the lower likelihood of developing sedation and dry mouth.
Taking moxonidine leads to a decrease in systemic vascular resistance and blood pressure. Moxonidine improves the insulin sensitivity index by 21% (compared to placebo) in patients with obesity, insulin resistance and moderate hypertension.