Release form and composition
Concor blood pressure tablets are available in several dosages - 5 and 10 mg of the active ingredient (bisoprolol fumarate). The light yellow, biconvex, heart-shaped tablets are scored on both sides for easy halving.
In addition to the main substance, the composition also includes auxiliary substances. These are corn starch, colloidal silicon dioxide, magnesium stearate, anhydrous calcium hydrogen phosphate, MCC. The shell consists of hypromellose, macrogol 400, dimethicone 100, yellow and red dyes iron oxide, titanium dioxide.
The drug Concor Cor has a reduced dose of the active component - 2.5 mg. This allows the drug to be prescribed to people for whom standard Concor is too strong, for example, with low blood pressure.
The main active ingredient of the drug is bisoprolol. Its main purpose is to protect the heart from the negative effects of catecholamines, including adrenaline. This allows you to effectively normalize blood pressure, restore optimal heart rate, reduce the risk of heart attack and other complications of hypertension. If beta blockers of previous generations in tablets and capsules affected the skeletal muscles, bronchi and pancreas, then Concor does not have such disadvantages, which makes it extremely safe.
How do beta blockers work for hypertension?
concor during pregnancy
Antiphospholipid syndrome, clinic, diagnosis, treatment Fig. 1. Causes of recurrent miscarriage
Fig 5. Placenta with antiphospholipid syndrome. Multiple placental thrombi usually accompany recurrent miscarriage. crm.region35.ru
Antiphospholipid syndrome (APS) is one of the most pressing multidisciplinary problems of modern medicine and is considered as a unique model of autoimmune thrombotic vasculopathy. The study of APS began about a hundred years ago in the works of A. Wassermann,
Antiphospholipid syndrome (APS) is one of the most pressing multidisciplinary problems of modern medicine and is considered as a unique model of autoimmune thrombotic vasculopathy.
The study of APS began about a hundred years ago in the works of A. Wassermann, devoted to the laboratory method for diagnosing syphilis. When conducting screening studies, it became obvious that a positive Wasserman reaction can be detected in many people without clinical signs of syphilitic infection. This phenomenon is called the “biological false-positive Wasserman reaction.” It was soon established that the main antigenic component in the Wassermann reaction is a negatively charged phospholipid, called cardiolipin. The introduction of radioimmunological and then enzyme-linked immunosorbent method (ELI) for the determination of antibodies to cardiolipins (aCL) contributed to a deeper understanding of their role in human diseases. According to modern concepts, antiphospholipid antibodies (aPL) are a heterogeneous population of autoantibodies that interact with negatively charged, less often neutral phospholipids and/or phospholipid-binding serum proteins. Depending on the method of determination, aPL are conventionally divided into three groups: those detected using IPM using cardiolipin, less often other phospholipids; antibodies detected using functional tests (lupus anticoagulant); antibodies that are not diagnosed using standard methods (antibodies to protein C, S, thrombomodulin, heparan sulfate, endothelium, etc.).
As a result of close interest in studying the role of aPL and improving laboratory diagnostic methods, the conclusion was that aPL are a serological marker of a unique symptom complex, including venous and/or arterial thrombosis, various forms of obstetric pathology, thrombocytopenia, as well as a wide range of neurological, skin, and cardiovascular disorders. Since 1986, this symptom complex began to be designated as antiphospholipid syndrome (APS), and in 1994, at an international symposium on aPL, it was also proposed to use the term “Hughes syndrome” - after the English rheumatologist who made the greatest contribution to the study of this problem.
The true prevalence of APS in the population is still unknown. Since aPL synthesis is possible and normal, low levels of antibodies are often found in the blood of healthy people. According to various data, the frequency of detection of aCL in the population varies from 0 to 14%, on average it is 2–4%, while high titers are found quite rarely - in approximately 0.2% of donors. APL is detected somewhat more often in elderly people. However, the clinical significance of aPL in “healthy” individuals (i.e., those without obvious symptoms of the disease) is not entirely clear. Often, with repeated tests, the level of antibodies that were elevated in previous determinations normalizes.
An increase in the incidence of aPL has been noted in some inflammatory, autoimmune and infectious diseases, malignant neoplasms, while taking medications (oral contraceptives, psychotropic drugs, etc.). There is evidence of an immunogenetic predisposition to increased synthesis of aPL and their more frequent detection in relatives of patients with APS.
It has been proven that aPL is not only a serological marker, but also an important “pathogenetic” mediator that causes the development of the main clinical manifestations of APS. Antiphospholipid antibodies have the ability to influence most of the processes that form the basis of the regulation of hemostasis, the violation of which leads to hypercoagulation. The clinical significance of aPL depends on whether their presence in the blood serum is associated with the development of characteristic symptoms. Thus, manifestations of APS are observed only in 30% of patients with a positive lupus anticoagulant and in 30–50% of patients with moderate or high levels of aCL. The disease develops mainly at a young age, while APS can be diagnosed in children and even newborns. Like other autoimmune rheumatic diseases, this symptom complex is more common in women than in men (5:1 ratio).
Clinical manifestations
The most common and characteristic manifestations of APS are venous and/or arterial thrombosis and obstetric pathology. With APS, vessels of any size and location can be affected - from capillaries to large venous and arterial trunks. Therefore, the range of clinical manifestations is extremely diverse and depends on the localization of thrombosis. According to modern concepts, the basis of APS is a kind of vasculopathy caused by non-inflammatory and/or thrombotic damage to blood vessels and ending with their occlusion. Within the framework of APS, the pathology of the central nervous system, cardiovascular system, dysfunction of the kidneys, liver, endocrine organs, and gastrointestinal tract are described. The development of certain forms of obstetric pathology tends to be associated with placental vascular thrombosis (Table 1).
Venous thrombosis, especially deep vein thrombosis of the lower extremities, is the most typical manifestation of APS, including at the onset of the disease. Thrombi are usually localized in the deep veins of the lower extremities, but can often occur in the hepatic, portal, superficial and other veins. Repeated pulmonary embolisms are typical, which can lead to the development of pulmonary hypertension. Cases of the development of adrenal insufficiency due to thrombosis of the central vein of the adrenal glands have been described. In general, arterial thrombosis occurs approximately 2 times less frequently than venous thrombosis. They are manifested by ischemia and infarctions of the brain, coronary arteries, and peripheral circulatory disorders. Thrombosis of the intracerebral arteries is the most common location of arterial thrombosis in APS. Rare manifestations include thrombosis of large arteries, as well as the ascending aorta (with the development of arcaortic syndrome) and the abdominal aorta. A feature of APS is the high risk of recurrent thrombosis. Moreover, in patients with the first thrombosis in the arterial bed, repeated episodes also develop in the arteries. If the first thrombosis was venous, then repeated thromboses, as a rule, are observed in the venous bed.
Damage to the nervous system is one of the most severe (potentially fatal) manifestations of APS and includes transient ischemic attacks, ischemic stroke, acute ischemic encephalopathy, episyndrome, migraine, chorea, transverse myelitis, sensorineural hearing loss and other neurological and psychiatric symptoms. The leading cause of central nervous system damage is cerebral ischemia due to thrombosis of the cerebral arteries, but there are a number of neurological and neuropsychiatric manifestations caused by other mechanisms. Transient ischemic attacks (TIA) are accompanied by loss of vision, paresthesia, motor weakness, dizziness, transient general amnesia and often precede a stroke by many weeks or even months. Recurrent TIA leads to multi-infarct dementia, which is manifested by cognitive impairment, decreased ability to concentrate and memory, and other symptoms nonspecific to APS. Therefore, it is often difficult to differentiate from senile dementia, metabolic (or toxic) brain damage and Alzheimer's disease. Sometimes cerebral ischemia is associated with thromboembolism, the sources of which are the valves and cavities of the heart or the internal carotid artery. In general, the incidence of ischemic stroke is higher in patients with damage to the heart valves (especially the left side).
Headaches are traditionally considered one of the most common clinical manifestations of APS. The nature of headaches varies from classic intermittent migraine to constant, unbearable pain. There are a number of other symptoms (Guillain–Barré syndrome, idiopathic intracranial hypertension, transverse myelitis, parkinsonian hypertonicity), the development of which is also associated with the synthesis of aPL. Patients with APS often experience veno-occlusive eye diseases. One of the forms of such pathology is transient loss of vision (amaurosis fugax). Another manifestation - optic neuropathy is one of the most common causes of blindness in APS.
Heart damage is represented by a wide range of manifestations, including myocardial infarction, damage to the valvular apparatus of the heart, chronic ischemic cardiomyopathy, intracardiac thrombosis, arterial and pulmonary hypertension. In both adults and children, coronary artery thrombosis is one of the main localizations of arterial occlusion due to overproduction of aPL. Myocardial infarction occurs in approximately 5% of aPL-positive patients, and it usually occurs in men under 50 years of age. The most common cardiac symptom of APS is damage to the heart valves. It ranges from minimal disturbances detected only by echocardiography (slight regurgitation, thickening of the valve leaflets) to heart disease (stenosis or insufficiency of the mitral, less often aortic and tricuspid valves). Despite its widespread occurrence, clinically significant pathology leading to heart failure and requiring surgical treatment is rarely observed (in 5% of patients). However, in some cases, very severe damage to the valves with vegetations caused by thrombotic layers, indistinguishable from infective endocarditis, can quickly develop. Detection of vegetations on the valves, especially if they are combined with hemorrhages in the subungual bed and “tympanic fingers”, creates complex diagnostic problems and the need for a differential diagnosis with infective endocarditis . Within the framework of AFS, the development of cardiac blood clots simulating myxoma has been described.
Renal pathology is very diverse. Most patients have only asymptomatic moderate proteinuria (less than 2 g per day), without renal dysfunction, but acute renal failure may develop with severe proteinuria (up to nephrotic syndrome), active urinary sediment and arterial hypertension. Kidney damage is associated mainly with intraglomerular microthrombosis and is defined as "renal thrombotic microangiopathy".
Patients with APS have clear and specific skin lesions, primarily livedo reticularis (occurring in more than 20% of patients), postthrombophlebitic ulcers, gangrene of the fingers and toes, multiple hemorrhages in the nail bed and other manifestations caused by vascular thrombosis.
In APS, there is damage to the liver (Budd-Chiari syndrome, nodular regenerative hyperplasia, portal hypertension), the gastrointestinal tract (gastrointestinal bleeding, splenic infarction, thrombosis of mesenteric vessels), and the musculoskeletal system (aseptic bone necrosis).
Characteristic manifestations of APS include obstetric pathology, the frequency of which can reach 80%. Fetal loss can occur at any time during pregnancy, but is somewhat more common in the second and third trimesters. In addition, the synthesis of aPL is associated with other manifestations, including late gestosis, preeclampsia and eclampsia, intrauterine growth retardation, and premature birth. The development of thrombotic complications in newborns of mothers with APS has been described, which indicates the possibility of transplacental transfer of antibodies.
Thrombocytopenia is typical for APS. Typically, the platelet count ranges from 70 to 100 x109/l and does not require special treatment. The development of hemorrhagic complications is rare and, as a rule, is associated with a concomitant defect in specific blood coagulation factors, kidney pathology, or an overdose of anticoagulants. Coombs-positive hemolytic anemia is often observed (10%), Evans syndrome (a combination of thrombocytopenia and hemolytic anemia) is less common.
Diagnostic criteria
The multi-organ nature of symptoms and the need for special confirmatory laboratory tests in some cases make it difficult to diagnose APS. In this regard, in 1999, preliminary classification criteria were proposed, according to which the diagnosis of APS is considered reliable when at least one clinical and one laboratory sign is combined.
Clinical criteria:
- Vascular thrombosis: one or more episodes of thrombosis (arterial, venous, small vessel thrombosis). Thrombosis must be confirmed using instrumental methods or morphologically (morphology - without significant inflammation of the vascular wall).
- Pregnancy pathology can have one of three options:
– one or more cases of intrauterine death of a morphologically normal fetus after 10 weeks of pregnancy;– one or more episodes of premature birth of a morphologically normal fetus before 34 weeks of pregnancy due to severe preeclampsia, or eclampsia, or severe placental insufficiency;
– three or more consecutive cases of spontaneous abortions before 10 weeks of pregnancy (with the exception of anatomical defects of the uterus, hormonal disorders, maternal and paternal chromosomal disorders).
Laboratory criteria:
- positive aCL class IgG or IgM in serum in medium and high titers, determined at least twice, with an interval of at least 6 weeks, using a standardized enzyme immunoassay;
- a positive lupus anticoagulant detected in plasma at least at an interval of at least 6 weeks using a standardized method.
Differential diagnosis
Differential diagnosis of APS is carried out with a wide range of diseases occurring with vascular disorders. It should be remembered that with APS there is a very large number of clinical manifestations that can imitate various diseases: infective endocarditis, heart tumors, multiple sclerosis, hepatitis, nephritis, etc. APS in some cases is combined with systemic vasculitis. It is believed that APS should be suspected when the development of thrombotic disorders (especially multiple, recurrent, with unusual localization), thrombocytopenia, obstetric pathology in young and middle-aged people in the absence of risk factors for the occurrence of these pathological conditions. It should be excluded in cases of unexplained thrombosis in newborns, in cases of skin necrosis during treatment with indirect anticoagulants, and in patients with a prolonged activated partial thromboplastin time in a screening study.
APS was first described as a variant of systemic lupus erythematosus (SLE). However, it was soon discovered that APS can also develop in other autoimmune rheumatic and non-rheumatic diseases (secondary APS). Moreover, it turned out that the connection between overproduction of aPL and thrombotic disorders is more universal and can be observed in the absence of reliable clinical and serological signs of other diseases. This served as the basis for the introduction of the term “primary APS” (PAPS). It is believed that approximately half of patients with APS suffer from the primary form of the disease. However, whether PAPS is an independent nosological form is not completely clear. Noteworthy is the high incidence of PAPS among men (the ratio of men to women is 2:1), which distinguishes PAPS from other autoimmune rheumatic diseases. Individual clinical manifestations or their combinations occur in patients with PAPS with varying frequencies, which is probably due to the heterogeneity of the syndrome itself. Currently, three groups of patients with PAPS are conventionally distinguished:
- patients with idiopathic deep vein thrombosis of the leg, which is often complicated by thromboembolism, primarily in the pulmonary artery system, leading to the development of pulmonary hypertension;
- young patients (up to 45 years) with idiopathic strokes, transient ischemic attacks, less often occlusion of other arteries, including coronary ones; the most striking example of this variant of PAF is Sneddon syndrome;
- women with obstetric pathology (repeated spontaneous abortions);
The course of APS, the severity and prevalence of thrombotic complications are unpredictable and in most cases do not correlate with changes in aPL levels and disease activity (in secondary APS). In some patients, APS may manifest as acute, recurrent coagulopathy, often in combination with vasculopathy, affecting many vital organs and systems. This served as the basis for identifying the so-called “catastrophic APS” (CAPS). To define this condition, the names “acute disseminated coagulopathy–vasculopathy” or “devastating non-inflammatory vasculopathy” have been proposed, which also emphasizes the acute, fulminant nature of this variant of APS. The main triggering factor for CAPS is infection. Less commonly, its development is associated with the abolition of anticoagulants or the use of certain medications. CAPS occurs in approximately 1% of patients with APS, but despite therapy, in 50% of cases it ends in death.
Treatment of APS
Prevention and treatment of APS are challenging. This is due to the heterogeneity of pathogenetic mechanisms, polymorphism of clinical manifestations, as well as the lack of reliable clinical and laboratory indicators to predict the recurrence of thrombotic disorders. There are no generally accepted international standards of treatment, and proposed recommendations are based mainly on the results of open-label drug trials or retrospective analyzes of disease outcomes.
Treatment with glucocorticoids and cytotoxic drugs for APS is usually ineffective, except in situations where the advisability of their use is dictated by the activity of the underlying disease (for example, SLE).
Management of patients with APS (as with other thrombophilias) is based on the prescription of indirect anticoagulants (warfarin, acenocoumarol) and antiplatelet agents (primarily low-dose acetylsalicylic acid - ASA). This is primarily due to the fact that APS is characterized by a high risk of repeated thrombosis, which significantly exceeds that of idiopathic venous thrombosis. It is believed that most patients with APS with thrombosis require prophylactic antiplatelet and/or anticoagulant therapy for a long time, and sometimes for life. In addition, the risk of primary and recurrent thrombosis in APS must be reduced by influencing such correctable risk factors as hyperlipidemia (statins: simvastin - simvastol, simlo; lovastatin - rovacor, cardiostatin; pravastatin - lipostat; atorvastatin - Avas, liprimar; fibrates: bezafibrate - cholestenorm ; fenofibrate - nofibal, grofibrate; ciprofibrate - lipanor), arterial hypertension (ACE inhibitors - capoten, sinopril, diroton, moex; b-blockers - atenolol, concor, egilok, betaloc ZOK, dilatrend; calcium antagonists - amlovas, norvasc, normodipine, lacidipine), hyperhomocysteinemia, sedentary lifestyle, smoking, oral contraceptives, etc.
In patients with high levels of aPL in the serum, but without clinical signs of APS (including pregnant women without obstetric pathology and medical history), one should limit the use of small doses of ASA (50–100 mg/day). The most preferred drugs are aspirin cardio, thrombo ACC, which have a number of advantages (convenient dosage and the presence of a shell that is resistant to the action of gastric juice). This form makes it possible to provide not only a reliable antiplatelet effect, but also to reduce the adverse effect on the stomach.
Patients with clinical signs of APS (primarily thromboses) require more aggressive anticoagulant therapy. Treatment with vitamin K antagonists (warfarin, phenylin, acenocoumarol) is undoubtedly a more effective, but less safe (compared to ASA) method of preventing venous and arterial thrombosis. The use of vitamin K antagonists requires careful clinical and laboratory monitoring. Firstly, this is associated with an increased risk of bleeding, and the risk of developing this complication due to its severity outweighs the benefit of preventing thrombosis. Secondly, in some patients, recurrence of thrombosis is observed after cessation of anticoagulant therapy (especially during the first 6 months after discontinuation). Thirdly, patients with APS may experience pronounced spontaneous fluctuations in the international normalized ratio (INR), which significantly complicates the use of this indicator for monitoring warfarin treatment. However, all of the above should not be an obstacle to active anticoagulant therapy in those patients for whom it is vitally necessary (Table 2).
The warfarin treatment regimen consists of prescribing a loading dose (5–10 mg of the drug per day) for the first two days, and then selecting the optimal dosage to ensure maintenance of the target INR. It is advisable to take every dose in the morning, before determining the INR. In older people, lower doses of warfarin should be used to achieve the same level of anticoagulation than in younger people. It is necessary to keep in mind that warfarin interacts with a number of medications, which, when administered in combination, both reduce (barbiturates, estrogens, antacids, antifungal and antituberculosis drugs) and enhance its anticoagulant effect (non-steroidal anti-inflammatory drugs, antibiotics, propranolol, ranitidine, etc.). Certain dietary recommendations should be given, since foods rich in vitamin K (liver, green tea, leafy vegetables - broccoli, spinach, Brussels sprouts, cabbage, turnips, lettuce) contribute to the development of warfarin resistance. Alcohol is avoided during warfarin therapy.
If warfarin monotherapy is insufficiently effective, combination therapy with indirect anticoagulants and low-dose ASA (and/or dipyridamole) is possible. This treatment is most justified in young people without risk factors for bleeding.
In case of excessive anticoagulation (INR>4) in the absence of bleeding, it is recommended to temporarily discontinue warfarin until the INR returns to the target level. In the case of hypocoagulation accompanied by bleeding, the administration of vitamin K alone is not enough (due to the delayed onset of action - 12–24 hours after administration); fresh frozen plasma or (preferably) prothrombin complex concentrate is recommended.
Aminoquinoline drugs (hydroxychloroquine - Plaquenil, chloroquine - Delagil) can provide quite effective prevention of thrombosis (at least in secondary APS due to SLE). Along with the anti-inflammatory effect, hydroxychloroquine has certain antithrombotic (suppresses platelet aggregation and adhesion, reduces thrombus size) and lipid-lowering effects.
The central place in the treatment of acute thrombotic complications in APS is occupied by direct anticoagulants - heparin and especially low-molecular-weight heparin preparations (Fraxiparin, Clexane). The tactics of their use do not differ from the generally accepted ones.
In CAPS, the entire arsenal of intensive and anti-inflammatory therapy methods used in critical conditions of patients with rheumatic diseases is used. The effectiveness of treatment to a certain extent depends on the ability to eliminate the factors that provoke its development (infection, activity of the underlying disease). The prescription of high doses of glucocorticoids for CAPS is not aimed at treating thrombotic disorders, but is determined by the need for treatment of systemic inflammatory response syndrome (widespread necrosis, adult distress syndrome, adrenal insufficiency, etc.). Pulse therapy is usually carried out according to a standard regimen (1000 mg methylprednisolone intravenously per day for 3–5 days), followed by glucocorticoids (prednisolone, methylprednisolone) orally (1–2 mg/kg/day). Intravenous immunoglobulin is administered at a dose of 0.4 g/kg for 4–5 days (it is especially effective for thrombocytopenia).
CAPS is the only absolute indication for plasmapheresis sessions, which should be combined with maximum intensive anticoagulant therapy, the use of fresh frozen plasma and pulse therapy with glucocorticoids and cytostatics. Cyclophosphamide (Cytoxan, Endoxan) (0.5–1 g/day) is indicated for the development of CAPS against the background of exacerbation SLE and to prevent “rebound syndrome” after plasmapheresis sessions. The use of prostacyclin (5 ng/kg/min for 7 days) is justified, however, due to the possibility of “rebound” thrombosis, treatment should be carried out with caution.
The administration of glucocorticoids to women with obstetric pathology is currently not indicated, due to the lack of data on the advantages of this type of therapy and due to the high frequency of side effects in the mother (Cushing's syndrome, diabetes, arterial hypertension) and fetus. The use of glucocorticoids is justified only in case of secondary APS against the background of SLE, since it is aimed at treating the underlying disease. The use of indirect anticoagulants during pregnancy is in principle contraindicated due to their teratogenic effect.
The standard for preventing recurrent fetal losses is small doses of ASA, which are recommended to be taken before, during pregnancy and after the birth of the child (at least for 6 months). During pregnancy, it is advisable to combine small doses of ASA with low molecular weight heparin preparations. During delivery by cesarean section, the administration of low molecular weight heparins is canceled for 2–3 days and resumed in the postpartum period, followed by a transition to indirect anticoagulants. Long-term heparin therapy in pregnant women can lead to the development of osteoporosis, therefore, to reduce bone loss, it is necessary to recommend taking calcium carbonate (1500 mg) in combination with vitamin D. It should be borne in mind that treatment with low molecular weight heparin is less likely to cause osteoporosis. One of the limitations to the use of low molecular weight heparins is the risk of developing an epidural hematoma, therefore, if there is a possibility of premature delivery, treatment with low molecular weight heparins is discontinued no later than 36 weeks of pregnancy. The use of intravenous immunoglobulin (0.4 g/kg for 5 days every month) has no advantages over standard treatment with ASA and heparin, and is indicated only if standard therapy is ineffective.
Moderate thrombocytopenia in patients with APS does not require special treatment. In secondary APS, thrombocytopenia is well controlled by glucocorticoids, aminoquinoline drugs and, in some cases, low doses of ASA. Treatment tactics for resistant thrombocytopenia, which poses a risk of bleeding, include the use of glucocorticoids in high doses and intravenous immunoglobulin. If high doses of glucocorticoids are ineffective, splenectomy is the treatment of choice.
In recent years, new antithrombotic agents have been intensively developed, which include heparinoids (heparoid lecheva, emeran, sulodexide - Wessel Due), platelet receptor inhibitors (ticlopidine, tagren, ticlopidine-ratiopharm, clopidogrel, Plavix) and other drugs. Preliminary clinical data indicate the undoubted promise of these drugs.
All patients with APS should be under long-term clinical observation, the primary task of which is to assess the risk of recurrent thrombosis and their prevention. It is necessary to control the activity of the underlying disease (in case of secondary APS), timely detection and treatment of concomitant pathologies, including infectious complications, as well as an impact on correctable risk factors for thrombosis. It has been established that prognostically unfavorable factors with regard to mortality in APS are arterial thrombosis, a high frequency of thrombotic complications and thrombocytopenia, and laboratory markers include the presence of a lupus anticoagulant. The course of APS, the severity and prevalence of thrombotic complications are unpredictable; unfortunately, there are no universal treatment regimens. The above-mentioned facts, as well as the multiorgan nature of symptoms, require the unification of doctors of various specialties to solve problems associated with the management of this category of patients.
N. G. Klyukvina , Candidate of Medical Sciences, Associate Professor of MMA named after. I. M. Sechenova, Moscow
www.rmj.ru
Rice. 2. Scheme of the pathogenesis of CAPS (modified and supplemented scheme according to RA Asherson [29]). Rice. 3. Treatment algorithm for CAPS, proposed at the 10th International Congress on APS in Taormina (Sicily, September 2002) [4].
old.consilium-medicum.com
Treatment of antiphospholipid syndrome
First of all, it is necessary to identify and treat the underlying disease that led to the occurrence of APS (systemic lupus erythematosus, systemic scleroderma, chronic diseases, tumors, etc.). This treatment will result in a decrease in blood antibodies to phospholipids.
Drugs that reduce blood clotting (antiplatelet agents), antihistamines (relieve allergic mood), antimalarial drugs are also prescribed - they combine an anti-inflammatory effect with the ability to suppress platelet aggregation, which also helps prevent the development of thrombosis.
If a very large amount of antibodies is detected in the blood and there is a threat of acute vascular thrombosis, then blood purification is carried out using hemosorption or plasmapheresis (efferent methods of treatment).
In pregnant women with APS, timely diagnosis, preparation and rational management of pregnancy using medications and efferent methods of therapy reduces the risk of complications both during pregnancy and in the postpartum period and promotes the birth of viable children.
The eco-center prescribes: proginova 3 tablets. per day, 200 mg in morning. 4 times vaginally) – Omega-3 (Vitrum cardio) 1 caps per day – Neuromultivitis 2 tablets. per day – Magne B6 3 tablets. per day – Folacin 1 tablet. per day – Clexane 0.6 once in the evening – Actovegin 2 tablets. in a day
With Clexane everything is clear - you need to monitor the hemostasigram. All the usual APTT, Prothrombin (Quick), INR, Fibrinogen, Thrombin time, D-dimer.
You will have to take the medications throughout the pregnancy, Clexane (Fraxiporin, Fragmin is the same thing) runs out 10 days before birth, and then after birth they continue to take it for another 2-4 weeks. In addition to injections, you can also take chirantil or cardiomagnyl tablets to thin the blood. You won't be able to bear it without these drugs. But it is necessary to do a complete hemostasiogram + D-dimer at least once a month. Actovegin will help to avoid fetal hypoxia (unfortunately, with these diseases it is usually inevitable), but it is usually given first by droppers or intravenous injections, then by tablets. Frlocin is folic acid also until the end of pregnancy. The rest is up to 16-18 weeks. A lot of money will be spent on medications and tests + the injections will make your stomach blue. But if you want a child, you must endure it. I have a similar situation with APS. From 2 weeks before the cesarean section, I gave live injections every day (first Fraxiparin, then Clexane, and then the hemostesiologist prescribed Fragmin; it is cheaper and can be given once a day, not 2) and took pills in huge doses. She endured nothing, gave birth to a daughter after 2 missed pregnancies and 4 years of ordeal.
Indications for taking the drug
Concor, which is often called “Concord”, is prescribed for a number of disorders in the functioning of the cardiovascular system. This:
- arterial hypertension;
- stable angina;
- cardiac ischemia;
- chronic cardiac failure.
Concor is also taken for low blood pressure accompanied by rapid heartbeat. The dosage is selected strictly individually. For example, it is believed that 2.5 mg of bisoprolol cannot lower blood pressure, but copes with other duties perfectly.
If a patient is diagnosed with hypertension, he may be recommended another medicine - Concor AM. This drug for high blood pressure contains 2 active ingredients at once - amplodipine and bisoprolol in an optimal ratio.
Concor AM tablets, in addition to bisoprolol (5 or 10 mg), include another active ingredient - amplodipine (5 or 10 mg)
Side effects of the drug Concor cor
From the nervous system: increased fatigue, dizziness, headache, sleep disturbances, depression may be observed (especially at the beginning of therapy), rarely - hallucinations (usually mild and disappear within 1-2 weeks), sometimes - paresthesia. On the part of the organ of vision: visual disturbances, decreased tear production (must be taken into account when wearing contact lenses), conjunctivitis. From the cardiovascular system: in some cases - orthostatic hypotension, bradycardia, AV conduction disturbances, decompensation of heart failure with the development of peripheral edema, at the beginning of treatment - deterioration of the condition of patients with intermittent claudication or Raynaud's syndrome. From the respiratory system: in isolated cases - shortness of breath (in patients prone to bronchospasm). From the gastrointestinal tract : in some cases - diarrhea, constipation, nausea, abdominal pain, increased activity of liver enzymes in the blood serum (AST, ALT), hepatitis. From the musculoskeletal system: in some cases - muscle weakness, cramps, arthropathy affecting one or more joints (mono- or polyarthritis). From the endocrine system: decreased glucose tolerance (with latent diabetes mellitus) and masking of signs of hypoglycemia, in some cases - increased TG levels in the blood, potency disorders. On the skin: sometimes - itching, skin hyperemia, increased sweating, rash. When treated with beta-adrenergic receptor blockers, hair loss, hearing impairment or tinnitus, weight gain, mood changes, short-term memory loss, allergic rhinitis, and priapism are observed in some cases.
Contraindications
The drug is contraindicated in the following cases:
- with cardiogenic shock;
- acute heart failure;
- metabolic acidosis;
- symptomatic bradycardia (low heart rate);
- 2nd – 3rd degree AV block;
- sick sinus syndrome;
- severe form of peripheral circulatory disorders;
- Raynaud's disease;
- untreated pheochromocytoma;
- respiratory tract diseases (bronchial asthma, bronchospasm);
- hormonal disorders.
You should avoid taking Concor if you are intolerant to any component of the drug and people under 18 years of age. In addition to absolute contraindications, there are also relative ones, in which Concor for blood pressure is prescribed with extreme caution and under constant medical supervision. This:
- diabetes complicated by significant fluctuations in blood glucose;
- strict dietary restrictions;
- desensitization;
- psoriasis;
- 1st degree AV block;
- peripheral arterial occlusive disease;
- general anesthesia.
During pregnancy and lactation with high blood pressure, Concor is prescribed only in severe conditions of the patient. Experts believe that the drug, like other beta blockers, can negatively affect the condition of the placenta and fetus. If the expectant mother nevertheless took the medicine during pregnancy, the baby is carefully monitored in the first days after birth, since the child’s pulse and blood glucose level may decrease to critical levels.
Special instructions for the use of the drug Concor cor
Concor Cor should not be used during pregnancy and breastfeeding due to the lack of reliable clinical data confirming the safety of the drug. During pregnancy, Concor Cor should only be recommended if the benefit to the mother outweighs the risk of side effects to the fetus. In exceptional cases, the use of bisoprolol during pregnancy should be discontinued 72 hours before the expected due date due to the possibility of bradycardia, hypoglycemia and respiratory depression in the newborn. If discontinuation of the drug is not possible, then after birth the newborn should be under medical supervision. Symptoms of hypoglycemia may occur during the first 3 days. In some cases, beta-adrenergic receptor blockers may cause the development or exacerbation of psoriasis. In patients taking β-adrenergic blockers, due to weakened adrenergic feedback regulation, anaphylactic reactions may be more severe. Due to the individual nature of reactions to the drug, the ability to drive vehicles or operate machinery may be reduced. To a greater extent, this applies to the initial stage of treatment and changes in the dose of the drug, as well as with the simultaneous use of alcohol. There are no clinical data on the effectiveness and safety of Concor Cor in children.
Adverse reactions to the drug
Although the medicine is usually well tolerated, in some cases adverse reactions to the components of the tablets occur. This:
- weakness, increased fatigue;
- sleep problems;
- depressive states;
- short-term memory lapses;
- hearing, vision, taste impairments;
- dermatological reactions;
- stomach cramps;
- intestinal disorders;
- circulatory disorders;
- heart failure.
It is worth considering that side effects are temporary and appear at the initial stage of treatment, completely disappearing after 2 weeks of therapy. This is evidenced by numerous reviews from doctors and their patients.
Overdose symptoms
For high blood pressure, Concor is taken once a day. If this treatment regimen is followed, an overdose is impossible. However, in a situation where for any reason the patient takes a large dose of the drug, serious problems may occur that require immediate medical attention. Signs of overdose are as follows:
- heartbeat instability (arrhythmia, tachycardia);
- a sharp decrease in heart rate (bradycardia);
- pronounced decrease in pressure;
- swelling of the limbs;
- difficulty breathing;
- change in skin color of limbs and nails;
- hypoglycemia;
- feeling of severe dryness in the mouth;
- weakness, loss of consciousness.
You cannot delay in providing assistance, since Concor reduces blood pressure very strongly, which can lead to a number of complications. The patient must be given a sorbent, which helps stop the absorption of the drug by the intestines and call an ambulance. Doctors will rinse the stomach and administer antidotes that reduce the effect of a large dose of Concor. These are drugs that have the opposite effect - “Atropine”, “Isoprenaline”, which affect blood pressure, increasing it.
Overdose of Concor Cor, symptoms and treatment
Symptoms: arrhythmia, ventricular extrasystole, severe bradycardia, AV block, marked decrease in blood pressure, acute heart failure, hypoglycemia, acrocyanosis, difficulty breathing, bronchospasm, dizziness, fainting, convulsions. Treatment: gastric lavage and administration of adsorbent drugs; symptomatic therapy: in case of developed AV block, intravenous administration of 1–2 mg of atropine, epinephrine or placement of a temporary pacemaker; for ventricular extrasystole - lidocaine (class IA drugs are not used); with a pronounced decrease in blood pressure, the patient should be in a position with the foot end of the bed raised; if there are no signs of pulmonary edema - intravenous plasma replacement solutions; if ineffective - administration of epinephrine, dopamine, dobutamine (to maintain chronotropic and inotropic effects and eliminate the pronounced decrease in blood pressure); for heart failure - cardiac glycosides, diuretics, glucagon; for convulsions - intravenous diazepam; for bronchospasm - β2-adrenergic stimulants by inhalation.
Admission rules
The scheme of how to take Concor depends on the patient’s health condition and the task assigned to the therapy. The maximum daily dose is 20 mg, but the decision about this amount of bisoprolol entering the body is made only in exceptional cases.
- On average, patients with hypertension are recommended to drink 5–10 mg of the drug per day. Sometimes the therapeutic course begins with 2.5, gradually increasing the dosage if well tolerated by the patient.
- If the patient's main diagnosis is heart failure, treatment begins with 1.25 mg of bisoprolol. Over time, the dose can be increased to 2.5 mg.
- Whether or not this drug reduces blood pressure depends on the dosage. Therefore, with low blood pressure, the dose should be minimal (1.25–2.5 mg).
- The dose can be reduced or increased only after consultation with the attending physician.
- There is no connection to food intake.
- The maximum antihypertensive effect is achieved after 3–4 hours and lasts throughout the day. The pressure becomes stable after 1–2 weeks of treatment.
- If after some time the pressure becomes normal and your health improves, treatment should not be interrupted. Abrupt withdrawal can lead to stroke, heart attack or hypertensive crisis.
The drug is taken once a day, which is very convenient, especially for forgetful patients
Concor not only lowers blood pressure, but also has some sedative effect, which disappears when you get used to the drug. For this reason, at the beginning of the therapeutic course (1.5 weeks), you should refrain from driving vehicles and performing work on complex mechanisms.
Pharmacological properties of the drug Concor cor
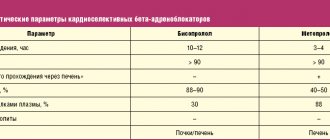
Bisoprolol (INN - bisoprololum) is a selective β1-adrenergic receptor blocker. When used in therapeutic doses, it does not have BSA and clinically significant membrane-stabilizing properties. Reduces plasma renin activity, reduces myocardial oxygen demand, and reduces heart rate (at rest and during exercise). By blocking β1-adrenergic receptors of the heart in low doses, it reduces the catecholamine-stimulated formation of cAMP from ACE, reduces the intracellular current of calcium ions, and has a negative chrono-, dromo- and inotropic effect (inhibits conduction excitability, slows down AV conduction). The antianginal effect is due to a decrease in myocardial oxygen demand as a result of a decrease in heart rate, a slight decrease in contractility, prolongation of diastole, and improved myocardial perfusion. When increasing the dose above the therapeutic one, it has a β2-adrenergic blocking effect. Concor Cor has a hypotensive effect due to a decrease in cardiac output, inhibition of renin secretion by the kidneys, as well as an effect on the baroreceptors of the aortic arch and carotid sinus. In case of hypertension (arterial hypertension), the effect occurs after 2–5 days, stable effect occurs after 1–2 months. With prolonged use, bisoprolol reduces increased peripheral vascular resistance. When used in average therapeutic doses, in contrast to non-selective β-blockers, it has a less pronounced effect on organs containing β2-adrenergic receptors (pancreas, skeletal muscles, smooth muscles of peripheral arteries, bronchi and uterus) and on carbohydrate metabolism, does not cause delay sodium ions (Na+) in the body. The severity of the atherogenic effect does not differ from the effect of propranolol. After taking the drug orally, bisoprolol is well absorbed from the gastrointestinal tract. Bioavailability is about 90% and is independent of food intake. The maximum concentration is reached after 1–3 hours. Binding to blood plasma proteins is about 30%. The effect of primary passage through the liver is insignificant (about 10%). About 50% of bisoprolol is biotransformed in the liver with the formation of inactive metabolites. The main metabolites found in blood plasma and urine do not exhibit pharmacological activity. The pharmacokinetics of bisoprolol is linear. Its concentration in blood plasma is proportional to the administered dose in the dose range from 5 to 20 mg. The maximum concentration in blood plasma is reached after 2–3 hours. Bisoprolol is distributed quite widely. The volume of distribution is 3.5 l/kg. Communication with blood plasma proteins is about 35%. The total clearance is 15.6 ± 3.2 l/h, with renal clearance being 9.6 ± 1.6 l/h. The half-life is 10–12 hours. Approximately 98% is excreted from the body in the urine, 50% unchanged, the rest in the form of metabolites, approximately 2% of the dose is excreted in the feces. No dose adjustment is required for patients with mild to moderate hepatic or renal impairment.
Interaction with other drugs
When treating with Concor, it is important to consider the compatibility with it of all medications taken additionally. It is strongly not recommended to take non-steroidal anti-inflammatory drugs simultaneously with Concor. The drug interacts poorly with some medications used in the treatment of cardiovascular diseases. This applies not only to products for lowering blood pressure from pharmaceutical companies, but even to traditional medicine recipes - decoctions, tinctures, infusions.
Therefore, the use of any medications during treatment with Concor should be agreed with the attending physician. In some cases, the specialist will recommend eliminating medications from the treatment regimen, in others he will reduce their dosage in order to increase the effectiveness of treatment.
Interactions of the drug Concor cor
With simultaneous use, Concor Cor may enhance the effect of antihypertensive drugs. With the simultaneous use of bisoprolol and reserpine, methyldopa, clonidine or guanfacine, a sharp decrease in heart rate is possible. When Concor Cor is used together with clonidine, digitalis preparations, and guanfacine, cardiac conduction disorders may develop. When Concor is used together with verapamil or diltiazem and other antiarrhythmic drugs, a decrease in blood pressure is possible, and the risk of developing or worsening bradycardia, AV block, cardiac arrest and heart failure increases (IV administration of calcium channel blockers and antiarrhythmic drugs during Concor therapy should be avoided Cor).Nifedipine can lead to a significant decrease in blood pressure. Phenytoin with intravenous administration and drugs for inhalation general anesthesia (hydrocarbon derivatives) increase the severity of the cardiodepressive effect and the likelihood of a decrease in blood pressure when used while taking bisoprolol. The effectiveness of insulin and oral hypoglycemic drugs may change during treatment with Concor Cor (masks the symptoms of developing hypoglycemia: tachycardia, increased blood pressure). The clearance of lidocaine and xanthines may decrease due to a possible increase in their concentration in the blood plasma, especially in patients with an initially increased clearance of theophylline under the influence of smoking. NSAIDs, corticosteroids and estrogens weaken the hypotensive effect of bisoprolol (Na+ retention, blockade of prostaglandin synthesis by the kidneys). With the simultaneous use of Concor Cor and sympathomimetics (including cough suppressants, eye drops and nasal drops), the effect of bisoprolol may be weakened. Diuretics, clonidine, sympatholytics, hydralazine and other antihypertensive drugs can lead to an excessive decrease in blood pressure. The effect of non-depolarizing muscle relaxants and the anticoagulant effect of coumarins may be prolonged during treatment with bisoprolol. Tri- and tetracyclic antidepressants, antipsychotics (neuroleptics), ethanol, sedatives and hypnotics increase CNS depression. Concomitant use with MAO inhibitors is not recommended due to a significant increase in the hypotensive effect. The treatment break between taking MAO inhibitors and bisoprolol should be at least 14 days. Non-hydrogenated ergot alkaloids increase the risk of developing peripheral circulatory disorders. Ergotamine increases the risk of developing peripheral circulatory disorders; sulfasalazine increases the concentration of bisoprolol in the blood plasma; Rifampin shortens the half-life. With the simultaneous use of ergotamine derivatives (including ergotamine-containing migraine drugs) and Concor Cor, the severity of peripheral circulatory disorders may increase. With the simultaneous use of Concor Cor and rifampicin, the half-life of bisoprolol may slightly decrease (increasing the dose of Concor is usually not required).
Analogs
Concor helps to effectively reduce blood pressure and has virtually no side effects. However, the price of the drug is not affordable for everyone. Selecting a high-quality alternative treatment that has a similar effect on the body will help you cope with the situation. Among the most popular of them are the following:
- Bisoprolol,
- Biol,
- Niperten,
- Coronal,
- Bisogamma and others.
A doctor who will take into account the characteristics of each medication will help you choose a substitute.