What role does calcium play in the body?
Calcium is an important trace element that affects metabolic processes in the body. It performs the following functions:
- affects the growth and development of bone tissue;
- participates in the process of blood clotting;
- regulates enzymatic activity;
- ensures good conductivity along nerve fibers to muscles;
- influences the contraction of muscle fibers and cardiac muscle;
- takes part in the production of hormones.
The health of bones and teeth depends on the calcium content in the body.
In addition, calcium ions help strengthen the vascular wall and increase the body's resistance to infections and allergic reactions.
How to determine
The amount of ionized calcium in blood serum can be determined by various methods of chemical analysis:
- colorimetry;
- turbidimetry;
- photometry.
The most economical and accurate method is ionometry. The study is carried out using special electrodes; the ions under study are deposited on the membrane of the electrodes, the amount of which is calculated in the analyzer.
Normal blood calcium levels
The content of ionized Ca in the blood depends on the age of the person. Average values normally range from 1.02 to 1.37 mmol/l. It is important to know that reference values for analysis may differ depending on the laboratory that performed the study. The table below shows the average calcium levels:
| Age category | Ca content, mmol/l |
| Newborns and children under 1 year | 1,02 – 1,37 |
| Children over 1 year to 14 years old | 1,28 – 1,32 |
| Adults | 1,16 – 1,3 |
Traditional methods
Herbal medicine is used after consultation with the attending physician, as an addition to the main treatment.
Hypocalcemia
Freshly washed leaves and young shoots of nettle are passed through a juicer. After which pure nettle juice is diluted with cold boiled water in a ratio of 1:3, the mixture is boiled for 3 minutes. Take 3 teaspoons/day for 3 doses (3 months).
St. John's wort herb (1 tablespoon) is poured with boiling water (1 glass) and kept in a water bath (15 minutes). Leave for 1 hour. Add 100 grams of honey and 1 tablespoon of lemon juice to the infusion. Drink the infusion 3 tablespoons/day for 3 doses (2 months).
Honey and butter in a 2:1 ratio are mixed and melted in a water bath, lemon juice is added to the cooled mixture. Drink 3 tablespoons/day for 3 doses.
Hypercalcemia
Herbal medicine is unlikely to be able to reduce the concentration of ionized calcium in the blood, but it is quite effective against calcifications on the walls of blood vessels.
1 tablespoon of crushed artichoke leaves is poured into 1 glass of boiling water and left to infuse. After the cooled infusion is drunk. Drink artichoke infusion once a day for 1 month.
10 grams of steelweed, dandelion, and burdock roots are poured with vodka (300 ml). They insist for 1 month. Drink 10 drops/day for 2 doses.
Causes of increased Ca ions in the blood and characteristic symptoms
Increased calcium (hypercalcemia) can occur in the following conditions:
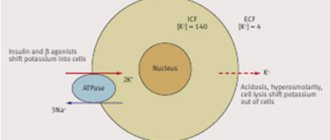
- metabolic disorders of hemostasis such as acidosis;
- increased production of Ca in newborns for no reason (Williams syndrome);
- excess vitamin D content;
- acute renal failure;
- malignant tumors and metastases in bone tissue;
- hereditary hypercalcemia;
- hyperparathyroidism, in which there is overproduction of the hormone of the parathyroid glands (parathyroid hormone);
- blood diseases: leukemia, lymphoma and others;
- benign tumor-like formations of the parathyroid gland;
- insufficiency of adrenal cortex functions;
- increased consumption of foods containing Ca.
Venous blood sampling is required to determine ionized calcium.
Hypercalcemia is characterized by the following symptoms:
- gradually increasing weakness, fatigue;
- decreased physical activity;
- dyspeptic disorders (feeling of nausea, vomiting);
- the appearance of thirst;
- convulsive twitching in the limbs;
- increased heart rate, cardiac arrhythmia.
With prolonged hypercalcemia, calcium deposition occurs in blood vessels, kidney and liver tissue. Heart failure may develop.
Decrease values:
- DiGeorge syndrome, or a genetic disorder in which the parathyroid and thymus glands are underdeveloped or absent;
- dysfunction of the parathyroid glands due to its autoimmune lesion or surgical intervention;
- decreased magnesium levels;
- decreased vitamin D levels;
- liver and kidney damage, which reduces albumin levels;
- damage to pancreatic tissue or pancreatic necrosis;
- long-term use of certain medications;
- massive blood transfusion.
Why does ionized Ca in the blood decrease and how does it manifest itself?
A decrease in calcium (hypocalcemia) can occur under the following conditions:
- with vitamin D deficiency;
- after extensive burns;
- with metabolic alkalosis;
- if the child has rickets;
- for renal pathologies, pancreatitis;
- if magnesium in the blood is low;
- in the postoperative period;
- with insufficient absorption of Ca in the intestine.
Ca ion deficiency may manifest itself as the following symptoms:
- Nervous excitability increases in patients;
- the emotional state becomes labile;
- characterized by migraine-like headaches and dizziness;
- osteoporosis, destruction of dental tissue and nails are noted;
- the skin becomes dry and the hair becomes brittle and weak;
- tachycardia appears;
- blood clotting is impaired - the period required to stop bleeding is extended.
Osteoporosis develops when there is a lack of calcium in the body.
Possible complications
Disorders of calcium metabolism without proper therapy can lead to very serious consequences.
A lack of ionized, active calcium in the blood serum can lead to such severe pathologies as:
- heart failure;
- blood clotting disorder;
- osteoporosis;
- impaired immunity;
- eye disorders (cataracts, lens dysfunction);
- neurological and mental disorders.
A very serious complication is tetany, in which death occurs when breathing or heartbeat stops.
Hypercalcemia can also lead to serious consequences:
- osteoporosis, pathological fractures;
- ICD;
- acute pancreatitis;
- intestinal obstruction;
- calcific uremic arteriolopathy;
- severe forms of arrhythmia.
The most dangerous condition, the symptom of which is an excess of ionized calcium in the blood, is considered a hypercalcemic crisis, in which death occurs from renal or heart failure.
Indications for testing for Ca ions
Biochemical analysis for ionized Ca is common in medical institutions. Since it carries important information on mineral metabolism in the body of both adults and children.
Indications for the study are the following conditions:
- signs of calcium deficiency or excess in the body;
- malignant tumors;
- diseases of the gastrointestinal tract;
- preoperative preparation;
- pathologies of the cardiovascular system;
- pain in muscles, bone tissue, muscle weakness;
- convulsive manifestations;
- impaired sensitivity in tissues;
- diseases of the urinary system;
- decrease in proteins in the blood.
When a patient is undergoing intensive therapy with intravenous administration of blood products and glucose-saline solutions, calcium levels are monitored daily or more often, as indicated.
Interpretation of results.
Calcium levels are assessed over time by a doctor in combination with other laboratory parameters and clinical data. Independent, isolated assessment leads to incorrect conclusions.
All laboratory services
Make an appointment through the application or by calling +7 +7 We work every day:
- Monday—Friday: 8.00—20.00
- Saturday: 8.00–18.00
- Sunday is a day off
The nearest metro and MCC stations to the clinic:
- Highway of Enthusiasts or Perovo
- Partisan
- Enthusiast Highway
Driving directions
Rules for preparing for analysis
To get a reliable result for calcium content, you must follow these rules:
- before taking the test, avoid heavy physical activity;
- do not drink alcohol or fatty foods the day before the test;
- The test must be taken strictly on an empty stomach, the last meal should be 12 hours before the test;
- Avoid smoking an hour before donating blood;
- Research cannot be carried out after instrumental examination methods and physical procedures.
It must be remembered that many medications can increase or decrease Ca in the body. Therefore, 1–2 weeks before the examination, you must stop taking medications. You should definitely consult your doctor on this issue. If it is not possible to discontinue medications, then the study form indicates which drug the patient is currently taking and in what dosage. This will help you conduct your research more accurately.
If symptoms of a calcium metabolism disorder in the body appear, you should immediately consult a specialist. You cannot self-diagnose and try to eliminate the symptoms yourself. This can lead to serious disorders in the body. Timely qualified diagnosis and correction of disorders will help prevent unwanted consequences and reduce the risk of complications.
Medications
Treatment of calcium metabolism disorders is a complex task. To prevent the transition of hypocalcemia to hypercalcemia and vice versa, doses of all drugs are selected individually, taking into account the disease that caused the pathology, gender, age, and calcium metabolism parameters.
During the treatment period, blood pH, main indicators of blood biochemistry and ionogram are constantly monitored. The duration of the course of treatment is also determined individually: they try to maintain blood calcium levels at the lower limits of normal for hypocalcemia and at higher limits for hypercalcemia.
Hypocalcemia
Monocomponent calcium preparations:
- Vitacalcin 1000 mg/day for 4 doses;
- Scoralite 750-1000 mg/day for 2-3 doses.
Combination drugs
- Natekal 2 tablets/day for 2 doses;
- Calcium D3 Nycomed 2 tablets/day for 2 doses.
Vitamin and mineral complexes:
- Complivit 2 tablets/day for 2 doses
- Multi Tabs 1 tablet/day for 1 dose.
Vitamin D preparations
- Ergocalciferol 3000 IU/day (40-45 days);
- Cholecalciferol 7500-15000 IU/day.
Hormonal drugs
- Parathyroidin 1.0-2.0 ml every other day
Hypercalcemia
Drugs that prevent bone resorption
- calcitonin 6-8 units/kg every 6-12 hours
- diphosphonates: (sodium etidronate 7.5 mg/kg IV drip; sodium pamidronate 15-45 mg/day; sodium alendronate 40 mg/day);
- potassium and sodium phosphates 1000-1500 mg/day.
Drugs that enhance calcium secretion
- Saline solutions and loop diuretics (0.9% saline or 5% glucose solution 4-8 liters/day + furosemide 20-40 mg every 3-6 hours).
Drugs that inhibit calcium absorption
- Glucocorticoids (prednisolone 30-60 mg/day)
- Cellulose sodium phosphate 15 g/day for 3 doses
4.What are the risks and what could interfere with the analysis?
What are the risks of a calcium blood test?
If you are taking a blood test for calcium, then possible risks may only be associated with taking blood from a vein. In particular, the appearance of bruises at the puncture site and inflammation of a vein or artery (phlebitis). Warm compresses several times a day will relieve phlebitis. If you are taking blood thinning medications, you may bleed at the puncture site.
What can affect the results of the analysis?
A calcium blood test may give inaccurate results if:
- You took calcium or vitamin D in any form (not only specially prepared preparations, but also, for example, dairy products) immediately before the test;
- You have had dialysis;
- You recently had a large blood transfusion.