With regard to drug treatment, there has long been an opinion among people that “we treat one thing, we cripple another.” And this is true, since most medications have a number of side effects that are detrimental to health. Even seemingly harmless intravenous administration of drugs can lead to inflammation of the veins.
This inflammatory process is called post-injection phlebitis. Let's take a closer look at what pathology is, why the veins become inflamed, and what therapeutic methods are most effective.
Post-injection inflammation of the veins: what is it?
Post-injection, or post-infusion, phlebitis is an inflammation of the venous walls, which is a complication of intravenous injection or infusion.
Among all types of vascular pathology, post-injection phlebitis is recognized as the most common form.
Administration of the drug through a vein causes vasospasm, provoking a narrowing of the venous lumen, as well as the penetration of infection, and leads to inflammation of the vein walls. This process is accompanied by a significant slowdown in blood circulation, deterioration of chemical indicators of blood composition, thinning of the tissues of the vascular walls, the formation of stagnation, and the detection of infectious agents in the plasma. All this increases the risk of blood clots, leading to a serious complication of phlebitis - thrombophlebitis.
Phlebitis caused by infusion is characterized by the following types:
- periphlebitis - inflammation of the subcutaneous tissue at the site of vessel damage;
- panphlebitis - damage to all venous layers;
- endophlebitis is a pathological change in the inner choroid.
Typically, inflammation of the veins after injection is localized on the arms or lower legs, but phlebitis can develop on any part of the body.
Causes of bruises
A bruise or hematoma in cancer patients is common, because chemotherapy is associated with introduction into the body through the destruction of the skin - intravenous administration of drugs, which is not always possible without hemorrhage into the skin and tissue around the vessel. Antitumor drugs change the composition of the blood, in particular the number of platelets, increasing bleeding, and also increase the likelihood of blood clots fivefold, while chronically existing varicose veins only double the formation of blood clots.
A malignant tumor changes the rheological properties of the blood, leading to the formation of blood clots. In every fifth patient, hidden thrombosis is detected before surgery. In intestinal and pancreatic cancer, excessive blood clot formation may be the first sign of a malignant disease. Chemotherapy, like the metastatic stage of any tumor, increases the likelihood of thrombosis by an average of five times. In the body of a cancer patient, two processes coexist simultaneously - excessive bleeding and increased coagulability.
Risk factors for thrombosis in cancer patients as in all “ordinary” people: high weight and age “after 40”, chronic vascular and heart diseases, diabetes mellitus, hypertension and, of course, varicose veins of the lower extremities.
These standard causes in an oncology patient are mixed with specific ones, that is, initiated by a malignant tumor and aggravated by the necessary treatment:
- compression of blood and lymphatic vessels by a tumor conglomerate;
- lymphostasis after removal of axillary lymph nodes, as a result of irradiation or removal of pelvic tissue with lymph nodes, as well as blockage of lymph outflow from the leg by metastatic inguinal ties;
- long-term use of hormonal drugs;
- stimulation of the red germ of hematopoiesis by erythropoietins;
- a permanently installed venous catheter for frequent drug infusions;
- restriction of mobility forced due to weakness;
- operations and radiation, as well as side effects of chemotherapy on blood vessels and blood cells.
To initiate a blood clot inside a vessel, a cancer patient needs only three reasons, and not necessarily all of them at once:
- damage to the inner lining of the vessel, which is common for intravenous chemotherapy;
- blood stagnation, which is called “poor circulation”, including as a result of tumor conglomerate;
- excessive coagulability along with impaired clot formation.
Book a consultation 24 hours a day
+7+7+78
Causes of inflammation of the walls of blood vessels
Post-injection phlebitis is formed due to damage to the vein during an intravenous injection or a catheter installed for infusions.
The risk of developing phlebitis depends on many factors. The main ones among them are the following:
- the size (length and diameter) of the needle through which the medicine was administered;
- use of low-quality raw materials for the manufacture of catheters and syringes;
- installation of a device (catheter) for a long time;
- failure to comply with sanitary standards during the procedure;
- ignoring aseptic rules;
- incorrectly calculated dose and high concentration of the administered drug;
- infection (candida, streptococci, staphylococci) due to failure to maintain sterility.
In addition, phlebitis of the vein in the arm can be caused by very rapid administration of a drug (especially solutions of calcium/potassium chloride, glucose, doxycycline hydrochloride) or too concentrated a substance.
After a catheter has been in the vein for a long time, there are frequent cases of infection, which further complicates the inflammation and course of phlebitis.
According to statistics, the development of phlebitis on the arm is most often provoked by self-installation of IVs at home (when removing patients from a drinking binge, ignoring hospital treatment, during urgent emergency care, etc.). Also at risk are people with drug addiction, who very often inject in places that are far from sterile. In such cases, the process of inflammation usually begins with damage to the inner layer of the vessel (endophlebitis) with further progression of the pathology.
How to prevent swelling
Of course, preventing swelling is much better than dealing with it later. Here are some recommendations for preventing hand swelling after an IV:
- When installing an IV, you need to relax as much as possible, because tense muscles are easier to damage.
- To prevent the drug from getting into the soft tissues, the medicine must be administered slowly and smoothly.
- To quickly stop bleeding, keep the cotton swab with alcohol in place for at least 10 minutes after the procedure.
- Make sure the needle and skin are sterile, especially for at-home IV drips.
- There are doctors with a “light hand”; it is advisable to go to them for the procedure. Such a doctor will insert the needle correctly and almost painlessly, which will reduce the risk of swelling. You can call such a professional to your home.
How does pathology manifest itself?
Phlebitis after intravenous drip or catheterization of veins manifests itself within 24 hours after the procedure and is accompanied by the following symptoms of venous inflammation:
- thickening due to the accumulation of blood at the injection site and protrusion of the vein outward (appears 2-3 hours after the injection;
- pain when moving a limb;
- stiffening (hardening) of soft tissues, detected upon palpation;
- the appearance of a sharp throbbing pain radiating to the fingers, shoulder, thigh (depending on where the injections were given);
- swelling and swelling of the injection area (appears after a few hours);
- redness of the affected area after 24 hours, followed by the appearance of a burgundy tint and blue discoloration;
- increasing swelling on day 2, swelling of the affected area, including surrounding tissues.
Ignoring the above symptoms of phlebitis leads to the fact that on the 3-4th day the limb stops bending/unbending at the knee/elbow joint, hyperemia and infiltration of the vascular walls develops, and body temperature increases (after some time it can reach 39-40°C).
In the future, the signs of venous inflammation only intensify:
- lymph nodes in the armpits and elbows become inflamed;
- suppuration of the walls of blood vessels forms, affecting nearby arteries.
With such an advanced stage of phlebitis, an operation to excise the pus is prescribed.
In addition, post-infusion phlebitis occurs against a background of general malaise, a noticeable decrease in physical activity, and pronounced pain.
Causes of swelling
The body’s unpleasant reaction to a drip has several causes, depending both on the professionalism of the health workers and on the physiological characteristics of the patient. Even by the speed of development of edema at the site of needle insertion, an experienced doctor can quickly determine the causes of its occurrence and immediately provide assistance.
Hitting the vein
Most often, swelling is caused by the injected drug getting into soft tissue. It happens that the needle passes right through the vein or does not enter it at all, then instead of blood, the medicine penetrates into the peripheral tissues, causing instant swelling. The hand swells literally before our eyes, increasing in size two to three times, pain, burning sensations, and numbness of the limb occur.
On the one hand, the inexperience of the health worker may be to blame: in order to find a vein and accurately hit it, certain skills are required. However, it happens that, by nature or from constant injections, the patient’s veins are difficult to palpate, then the introduction of an IV becomes a real test for all participants in the procedure. Swelling if the medicine gets under the skin is eliminated quickly enough and does not pose a serious threat.
Abscess
The situation is much worse with abscesses that arise as a result of violation of the basic rules of asepsis. Any little thing can cause the formation of a purulent cavity under the skin: poor treatment of the skin at the site of needle insertion, insufficient sterility of consumables, non-compliance with the sanitary and anti-epidemiological regime in the treatment room.
Subcutaneous inflammation does not occur as rapidly as edema, so it will not be detected earlier than a few hours after the intravenous injection. Symptoms of an abscess include redness and swelling of the arm in the area of the injection, accompanied by pain, as well as an increase in body temperature. The inflammatory process threatens serious complications, so at the first signs it is necessary to urgently consult a surgeon.
Allergy
When using a dropper, swelling of the arm can also be caused by an allergy to the drugs used for injection. Externally, an allergic reaction manifests itself in the form of pinpoint redness like “urticaria” or tension and itching of tissues, as with Quincke’s edema. The first symptoms appear within half an hour and intensify progressively, which in itself is an alarming “bell”.
Most often, allergies are observed with the administration of certain types of antibiotics, analgesics, and sulfonamide drugs. Typically, patients warn doctors about contraindications for medications known to them, but there are situations when the patient is not aware of individual intolerance to a particular drug.
Diagnostic methods
If you notice the above symptoms of phlebitis, you should immediately contact a medical facility. A phlebologist treats vascular pathologies.
An experienced specialist, with a thorough visual examination, based on the patient’s complaints and the presence of pronounced signs of post-injection phlebitis, will be able to make a diagnosis.
However, to establish an accurate diagnosis for venous inflammation (phlebitis is often confused with phlegmon of the extremities), additional examinations are necessary:
- general blood and urine analysis;
- blood test for coagulation;
- X-ray and ultrasound of the affected area.
Finding out the full clinical picture of phlebitis will help the specialist prescribe timely and competent treatment, and the patient will avoid severe complications of venous inflammation.
In what cases is radical therapy required?
If drug therapy does not give positive results in the fight against phlebitis and the disease lasts for a long time, then surgical intervention would be a reasonable solution. This occurs when the affected area is inflamed, suppuration begins, blood clots form, and post-injection thrombophlebitis occurs.
The operation is performed in a hospital setting. Under local anesthesia, the patient has the resulting suppuration removed. The next day after surgery, it is recommended to wrap your arm with an elastic bandage and try not to disturb the limb.
- Phlebitis of the veins on the arm: treatment, causes, prevention
If phlebitis, especially its acute form, is not treated, a complication of the inflammation process (for example, blood poisoning) may occur and lead to death.
Methods for treating phlebitis
Treatment of post-injection phlebitis is mainly carried out using conservative therapy, but in difficult cases they resort to more radical methods - surgery.
The choice of treatment method for phlebitis directly depends on how much time has passed since the first signs of venous inflammation were detected. If the patient consults a doctor 1-3 days after the onset of phlebitis, a medicinal method of treatment is used.
To avoid complications of inflammation of the venous walls (phlegmon/thromboembolism), treatment of post-infusion phlebitis is carried out in a hospital setting under the mandatory supervision of a vascular surgeon, especially if an acute period of the disease is detected.
Conservative treatment for phlebitis is prescribed for the purpose of antibacterial treatment and detoxification of the affected area, as well as stopping the source of inflammation, increasing blood circulation and restoring venous walls.
For the treatment of post-injection phlebitis, medications are prescribed:
- non-steroidal anti-inflammatory drugs - Ibuprofen, Butadione, Nimesil, etc.;
- angioprotectors that strengthen blood vessels and increase blood flow - Troxevasin, Heparin;
- indirect anticoagulants to reduce blood viscosity and prevent the formation of blood clots - Aspecard, Warfarin;
- fibrinolytics for dissolving blood clots (in complicated cases) – Urokinase, Streptokinase;
- antibacterial drugs - sulfonamides, tetracyclines, macrolides - to eliminate the risk of blood poisoning.
Treatment of phlebitis with the above-mentioned medications is carried out using tablets, topical preparations (ointments/gels/creams), as well as intramuscular and intravenous injections.
If the inflammation during phlebitis is very severe, then they resort to endolymphatic insertion of a catheter needle to accelerate the therapeutic effect of the drugs.
Gauze bandages with a silver solution, heparin ointment, and semi-alcohol compresses are used as local medications to eliminate phlebitis.
When a patient contacts you early (on days 1-2) for phlebitis, physiotherapeutic procedures are often prescribed. However, when inflammation of the veins intensifies (usually on the 3rd day), hyperthermic procedures are strictly contraindicated. It is allowed to apply cold to the damaged area.
If drug treatment for phlebitis is ineffective, they resort to the surgical method. This occurs when the affected area becomes even more inflamed, suppuration begins and blood clots form. The operation for phlebitis of the veins in the arms is performed in a hospital under local anesthesia and is reduced to removing the formed abscesses. The recovery period after such manipulation lasts about 2-3 weeks.
Doctors recommend wrapping the limb with an elastic bandage on the second day after surgery, as well as ensuring rest and placing the affected arm (leg) on an elevated surface.
Please note that if a surgical incision is made, physiotherapeutic procedures for the treatment of inflammation of the veins in the arms are prohibited.
Ignoring the treatment of post-infusion phlebitis or self-medication is unacceptable, since a complication of the inflammation process is possible, threatening the death of the patient.
Introduction
Radiofrequency and cryothermal catheter ablation are currently widespread types of non-drug treatment for cardiac arrhythmias, becoming a real alternative to drug therapy.
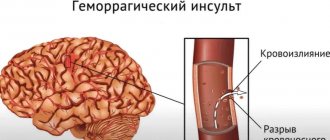
A mandatory element of any catheter interventions is transvascular access, which includes puncture of the femoral and, less commonly, subclavian, cubital or jugular veins. Currently, for intravascular access, the right and/or left femoral veins are usually punctured [1]. As is known, any catheter intervention is associated with a certain risk of complications from the site of vessel puncture. Among the complications associated with catheter ablation, thrombosis of the puncture site of the femoral veins deserves special attention. This is due to the potential risk of developing thromboembolic complications caused by the proximal localization of venous thrombosis (VT) [2].
Significance of the problem and risk factors.
According to various international observations, the incidence of thrombosis of the puncture site of the femoral veins after catheter interventions ranges from 0.3 to 3% [1, 3], but to date this problem has not been sufficiently studied. Statistical data is contradictory, which is due to the observational nature of the studies, since along with studies in which all patients were examined after interventions, there were studies in which verification of the puncture site was provided for in patients only in the presence of clinical symptoms [4-10, 13 ]. In 2017, a meta-analysis was conducted that combined data from publications from 1987 to 2013. on the study of venous thromboembolic complications (VTEC) in patients after electrophysiological studies (EPI), radiofrequency (RFA) and cryothermal catheter ablation, the results of which are presented in table. 1 and 2
Table 1. Studies to identify symptomatic VTE Note. Here and in the table. 2: LMWH - low molecular weight heparin.
Table 2. Asymptomatic VTE in patients after catheter ablations [3].
As mentioned earlier, the importance of VTEC associated with catheter electrophysiological interventions is due to their extremely high potential risk for the health and life of the patient: the most embologenic include thrombosis of the femoral and iliac veins, as well as the inferior vena cava [2].
The main links in the pathogenesis of thrombosis of the puncture site of the femoral veins after catheter interventions correspond to Virchow's triad: blood stasis, endothelial damage and hypercoagulation. And the immediate causes may be damage to the endothelium of the vascular wall during vein puncture and the need to immobilize patients after catheter intervention [14, 15].
Risk factors for the development of VTEC in patients after catheter interventions.
The discussed category of thrombosis is rarely mentioned in expert documents and recommendations due to the lack of data obtained from specially designed studies. The 2021 ISTH recommendations on the diagnosis, treatment and prevention of VTEC, regardless of catheter interventions, suggest focusing on two aspects: 1) whether this episode of VT is the first or repeated for the patient and 2) whether there is a reversible factor that led to the development of thrombosis, on the basis of which VTs are differentiated as provoked or unprovoked [16].
Clinical risk factors for the development of VT are age over 40 years, arterial hypertension, heart failure, varicose veins of the lower extremities, history of VTEC, diabetes mellitus, obesity, autoimmune processes, cancer (the greatest risk in the presence of metastases, as well as after chemotherapy), taking estrogen-gestagen drugs (as contraceptives or hormone replacement therapy) and many others [2]. Some of these factors are modifiable and, therefore, in most cases can be influenced and thereby reduce the risk of possible thromboembolic events before planned catheter intervention. However, a number of clinical factors, such as age, history of VTEC, are non-modifiable.
Non-modifiable factors also include thrombophilia, the concept of which is understood as a congenital or acquired pathological condition characterized by a tendency to increased blood clotting and, accordingly, an increased risk of blood clots [2]. The following have a high thrombotic risk: deficiency of natural anticoagulants (antithrombin III, proteins C and S), Leiden and prothrombin mutations, antiphospholipid syndrome. Also shown is the connection between the development of VTEC and polymorphisms in the folate cycle genes: methylenetetrahydrofolate reductase (MTHFR), reductase (MTRR) and methionine synthase (MTR), which is important as a risk factor for VTEC in the case of hyperhomocysteinemia. However, genetically determined disorders of the folate cycle are unlikely to have independent clinical significance due to their low prevalence in the population. The search for congenital thrombophilias is justified in young people (under 50 years), especially with repeated VTECs and in cases where it was not possible to find the factors that directly provoked thrombosis [2].
Factors such as the duration of the intervention and the “total” diameter of the introducers (the total diameter of the “puncture hole”) may also play a role in the pathogenesis of VAT after catheter interventions. These factors were studied in a prospective study by G. Moubarak et al. [12]. The study included 220 patients who underwent intracardiac EPI or RFA in the right side of the heart. During the procedure, all patients underwent puncture of the right femoral vein with the installation of 1 to 3 introducers. The average duration of the procedures was 45 minutes. All patients, 6 hours after catheter intervention, underwent duplex ultrasound scanning (USD) of the femoral vein puncture site, which revealed VT in 5% of cases: in 7 cases, parietal VT and in 4 cases, floating VT. It is important to note that clinical manifestations of thrombosis were not observed in any of the patients. As factors associated with the development of VT, the authors indicated procedure time (90 minutes versus 45 minutes) and the total diameter of the sheaths used (13 French versus 12 French).
Diagnosis of VTEC after catheter interventions.
In accordance with the position of experts, reflected in the recommendations of the International Society on Thrombosis and Haemostasis (ISTH) 2016 [16], for a patient with clinical manifestations that allow one to suspect VT, the diagnostic algorithm consists of assessing the clinical probability of thrombosis development , determining the level of D-dimer and performing ultrasound scanning of blood vessels. In patients with a low or moderate likelihood of developing VT, a normal D-dimer level allows one to exclude thrombosis and additional diagnostics are not indicated in such cases. In patients with a low and moderate likelihood of developing VT, but with an increase in D-dimer levels, as well as in patients with a high pre-test probability of developing VT, ultrasound scanning is recommended.
Patients scheduled for intracardiac EPS or RFA may represent a fairly heterogeneous population in terms of clinical risk factors. Along with young patients, there are patients in whom a combination of clinical factors (arterial hypertension, heart failure, diabetes mellitus, oncological pathology) and technical aspects of the procedure itself (its duration, large total diameter of the puncture hole, prolonged immobilization) can increase the risk of complications [ 2, 12]. However, the low incidence of VTEC development described in the literature allows us to classify intracardiac EPI and RFA as interventions associated with a low risk of VTEC development [3–13].
The role of determining D-dimer levels in the diagnosis of VT in patients after catheter ablations is controversial. It is not possible to focus on the level of D-dimer in this category of patients to exclude or confirm VT, since despite its high sensitivity this indicator has low specificity [2] and can be increased, including against the background of parietal and intermuscular hematomas, which are quite often form in patients in the puncture area.
The issue of routine blood testing to determine D-dimer levels in all patients before RFA has not been specifically studied. As mentioned earlier, patients undergoing catheter interventions, in most cases, belong to a group with a low likelihood of developing VTEC. According to modern international recommendations of the American College of Chest Physicians (ACCP) [17], in such patients, if they have normal D-dimer levels, further examination to further exclude VT is not indicated due to the low probability of VTEC. However, the situation remains unclear in the case of performing an intervention in patients with a moderate/high risk of VT (elderly patients with a history of VTEC, in the presence of oncological pathology). In such clinical situations, the low specificity of D-dimer is also likely to limit its diagnostic value. Nevertheless, the question of the possibility of identifying a patient with an increased risk of thrombosis after interventions is of scientific and practical interest. Assessing the predictive value of D-dimer in relation to thrombotic complications in comorbid, elderly patients, and patients with risk factors for VT requires further study [17, 18].
The main clinical manifestations of venous thrombosis of the lower extremities are pain, swelling, and discoloration of the skin. However, the peculiarity of the localization of VT in patients after catheter interventions, namely in the area of the iliofemoral segment, often determines their asymptomatic nature. In such cases, the only way to verify VT is to perform ultrasound scanning: when thrombi form in the lumen of the vein, structures of varying echogenicity, density and degree of occlusion are visualized [19, 20]. A number of studies [11–13] have shown that when performing ultrasonography, thrombosis of the puncture site of the femoral veins in patients after RFA was verified in 0–0.75% of cases, while none of them was accompanied by clinical symptoms.
It is likely that the incidence of asymptomatic VT may be higher than that previously indicated, since in a number of studies, ultrasonography was performed on patients only in the presence of symptoms, and this fact does not allow us to speak about the true frequency of VT after catheter interventions [3–10].
Prevention of VTEC in patients after catheter interventions.
Important aspects in the prevention of VTEC in patients after catheter interventions are the earliest removal of venous introducers, the minimum duration of use of pressure bandages (if necessary) at the site of venous puncture, as well as early activation of patients [3, 12].
When performing intracardiac EPI and RFA in patients without atrial fibrillation (AF), the intraoperative use of unfractionated heparin (UFH) is recommended only in cases of long surgical time, as well as in cases of high risk of VTEC [3, 21]. In a study by M. Scheinman et al. [6] UFH was not used during ablations, and the rate of VT was 0.13%; in a number of other studies that included the administration of heparin during the intervention [4, 5], the incidence of VT was 0.3–0.56%. However, the small number of studies does not allow us to compare the incidence of VT at the puncture site with and without intraoperative heparin administration.
Thus, in patients without AF and, therefore, without indications for long-term therapy with oral anticoagulants, adequate administration of heparin during catheter intervention is the key to the absence of VT of the puncture site.
In patients with AF who undergo cryo- or radiofrequency catheter ablation of the pulmonary vein ostia, the basis for preventing the occurrence of VT is both intra- and postoperative anticoagulant support [3]. During ablation, these patients need to create an anticoagulation background using UFH while maintaining the target AST level for at least 300 s. These recommendations also apply to patients who received anticoagulant therapy (ACT) before surgery [3, 21–24].
In a study by L. Haman et al. In order to prevent VTEC in patients undergoing radiofrequency exposure to the left side of the heart for AF, subcutaneous administration of low molecular weight heparin (LMWH) was considered in a therapeutic dose based on the patient’s body weight for the first 1-2 days; subsequently, antiplatelet therapy with acetylsalicylic acid (ASA) was prescribed at a dose of 200 mg/day for up to 1 month (it should be noted that, in accordance with the recommendations of that time, the prescription of ASA was provided for, but currently not). During observation, 3 (0.75%) of 400 patients were diagnosed with VT based on clinical manifestations [11].
ACT in the treatment of VTEC in patients after catheter interventions.
No special studies have been conducted to determine treatment tactics for VTEC in patients after catheter interventions, and there are no indications of a treatment algorithm in the current recommendations. Also, the group of patients we indicated was not included in studies or registries with either warfarin or new oral anticoagulants (NOACs).
As mentioned earlier, the formed VT at the site of femoral vein puncture after catheter intervention has a high potential risk for the patient’s life. That is why, in case of its verification, an ACT is required.
The most studied and widely used vitamin K antagonist is warfarin, the effectiveness of which in the treatment of VTEC has been convincingly proven in numerous clinical studies. However, long-term therapy with warfarin is associated with a number of difficulties, such as the need to select an individual dose of the drug, the need for constant (at least once a month) laboratory monitoring of the level of anticoagulation - international normalized ratio (INR), multiple food and drug interactions, the influence of genetic characteristics of patients (carriage of CYP 2 C 9
and
VKORC 1
) on the anticoagulant effect and the risk of hemorrhagic complications. Restrictions in the use of warfarin stimulated the development and introduction of new oral anticoagulants, the action of which is aimed at various points of application in blood coagulation:
1. Direct thrombin inhibitors: dabigatran etexilate.
2. Direct factor Xa inhibitors: rivaroxaban, apixaban, edoxaban.
There have been no direct comparisons of drugs from the NOAC group in the treatment of VAT not associated with catheter interventions. Each of the studies compared dabigatran, rivaroxaban and apixaban with enoxaparin and/or warfarin, so it is not justified to talk about the benefits of any one of the NOAC drugs. It is important to note that despite the narrow therapeutic window and the difficulties of laboratory monitoring, warfarin continues to be a widely prescribed drug. In the presence of oncological pathology, in accordance with modern recommendations, the use of LMWH is preferable.
For a long time, standard therapy for VT included the prescription of parenteral anticoagulants (LMWH in a therapeutic dose) followed by a transition to oral administration of vitamin K antagonists for at least 3 months. Modern recommendations for the treatment of VAT not associated with catheter interventions suggest the possibility of starting treatment with direct oral anticoagulants: in the case of rivaroxaban and apixaban - without prior use of LMWH, in the case of dabigatran and edoxaban - after a short initial course of parenteral anticoagulants [17].
Patients without AF do not require long-term ACT. However, in the case of the formation of VT, anticoagulants are required. However, as stated previously, the preferred drug has not been studied, and the duration of therapy remains a matter of debate.
For patients with AF, an important feature is the initial need for constant ACT. Updated ACCP guidelines advocate the preferred use of NOACs in these patients [17]. However, it is important to note the fact that the therapeutic dose of rivaroxaban for VT is higher than the prophylactic dose prescribed to patients with AF: 15 mg twice a day for 3 weeks, followed by a transition to taking the drug at a dose of 20 mg/day. However, dose adjustment of rivaroxaban according to glomerular filtration rate (GFR) is not taken into account. For apixaban, the dosage regimen for the treatment of VT also differs from the prophylactic dose in patients with AF: during the first week, the drug is prescribed at a dose of 10 mg twice a day, followed by a transition to a dosage of 5 mg twice a day. Thus, the therapeutic dose of these drugs is higher than that prescribed to patients with AF for preventive purposes. However, there are no data on dose adjustment of NOACs in the treatment of VT in current recommendations.
In our review, we have repeatedly pointed out that proximal VT is a potentially life-threatening complication. In this regard, for patients with AF, if a VT is detected at the site of femoral vein puncture after catheter interventions, it is advisable to switch to a therapeutic dose of the drug and resume the prophylactic dose after lysis of the thrombus under ultrasound control. However, to date this issue has not been studied.
The question of the timing of continuation of ACT in patients with AF after catheter ablation for the purpose of preventing VTEC was studied in a study by L. Prudente et al. [9]. The researchers evaluated different timing of enoxaparin therapy in 539 patients with AF who underwent RFA of the pulmonary veins. All patients were stopped taking warfarin 4 days before the intervention under the control of INR (the target level during drug withdrawal is less than 2.0). During ablation, UFH was administered at a dose of 130 U/kg body weight and then 23 U/kg every 1 hour 30 minutes under the control of AST levels (target level - 300-350 s). 4 hours after the procedure, ACT was resumed in all patients with warfarin against the background of subcutaneous administration of LMWH at a therapeutic dose of 1 mg/kg. Three regimens of LMWH therapy after ablation were considered: 1) at a dose of 1 mg/kg every 12 hours for 5 days; 2) at a dose of 1 mg/kg every 12 hours for 3 days; and 3) 0.5 mg/kg every 12 hours for 5 days. Based on the comparable frequency of VT after interventions, the authors concluded that the administration of enoxaparin at a dose of 0.5 mg/kg every 12 hours for 5 days is as effective as a dose of 1 mg/kg every 12 hours for the same period.
Traditional methods of treatment
To speed up the recovery process for post-injection phlebitis, alternative medicine is often used - ointments and compresses based on components of plant origin. However, treatment of inflammation of the vascular walls can be carried out only after consultation with a doctor and testing for the absence of an allergic reaction.
We suggest using the most effective recipes that relieve venous inflammation:
- Honey compress. Lubricate the area of inflammation with liquid honey and wrap it with natural fabric (linen, chintz).
- Applying cabbage leaf. Crumple a cabbage leaf thoroughly washed and scalded with boiling water, spread with honey and apply to the site of inflammation. Secure with a bandage.
- A decoction of beet tops relieves inflammation well. Pour 50 g of dried red beet leaf into 1 liter of boiling water. Let it brew for an hour. Take 150 ml of infusion in the morning on an empty stomach.
- A decoction of currant or rowan leaves. Take the raw material brewed using the above method for venous inflammation 2-3 times a day, 100 ml.
Please note that traditional medicine for inflammation of the vascular walls after injection are additional preventive measures and cannot replace the main treatment of phlebitis with medications.
Call your doctor immediately if you have:
- there was severe pain in the lower abdomen (stomach) when urinating;
- I can't urinate.
These symptoms may indicate the need for reinsertion of the catheter.
- Monday to Friday from 9:00 to 17:00 call _______________.
- After 5:00 p.m., and on weekends and holidays, call 212-639-2000 and ask to speak with the urologist on call.
If you are unable to come to Memorial Sloan Kettering (MSK), you can have a catheter installed by your local urologist or your nearest urgent care center. Tell them that you recently had prostate surgery.
If you see your local urologist or the nearest emergency room for any reason related to your prostate surgery, tell your MSK doctor the next business day.
to come back to the beginning
Preventive measures
Not a single patient is immune from the post-injection form of phlebitis. Therefore, every person must take responsibility for their health and lead a healthy lifestyle.
What to do to avoid the formation of phlebitis. To prevent vascular inflammation, doctors recommend the following measures:
- regular walks in the fresh air at any time of the year;
- rejection of bad habits;
- daily physical activity (jogging, gymnastic elements, aerobic and cardio exercise);
- adherence to the principles of proper nutrition;
- exclusion from the diet of fatty, salty, spicy foods;
- adherence to a daily routine (full rest and sleep are required).
If you are undergoing treatment using intravenous injections and droppers, lubricate the needle insertion site with venotonics (Venoruton, Troxevasin, etc.) to prevent inflammation of the vascular walls.
Remember that phlebitis does not forgive a frivolous attitude. Therefore, if you suspect venous inflammation, immediately seek help from specialists. Timely measures taken to eliminate phlebitis guarantee complete recovery.
Possible complications
If you do not give importance to the swelling of the hand after an IV and do not report the painful sensations to the doctor, a harmless hematoma can develop into a serious pathology.
- Phlebitis occurs when the wall of a vessel becomes inflamed due to injury from a needle or medication. Phlebitis can be caused by an allergic reaction to the drug. Symptoms of the pathology: the skin turns red and the temperature in the damaged area rises; Red stripes appear along the line of the vein, the arm is very sore and swollen. The disease is treated on an outpatient basis.
- Thrombophlebitis is the blockage of a blood vessel by a blood clot. The cause of the pathology is also damage or infection in the vein. The hand swells, the needle insertion site hardens, the skin turns red and the temperature rises. Blood clot rupture is especially dangerous, so it is important to immediately consult a doctor at the first symptoms.
- Thromboembolism is a blockage of a vein by a blood clot that interferes with blood flow. The symptoms are as follows: the formation of a purulent lump, severe swelling of the arm, throbbing pain, fever. The disease is treated with ointments and antibiotics, which provoke the opening of the cone.
- Cellulitis is a purulent inflammation that appears as a result of improper insertion of a needle into a vein, as a result of which the medicine enters the fatty layer. The source of the disease is not limited, it spreads throughout the tissues. Symptoms: intoxication, severe pain and swelling, severe fever. At first the infiltrate is hard to the touch, but gradually softens. This disease requires emergency surgery and antibiotics.
If symptoms such as fever, sharp throbbing pain and purulent formations appear, you should immediately consult a doctor. Self-medication may lead to the development of one of the described pathologies.
Sexual activity
You can resume sexual activity __________________.
You can start taking erection medication the evening of the day your catheter is removed. You may need to take one of these medications daily for 1 year after surgery.
Your doctor or nurse will give you information about your medication schedule. Follow this schedule until your postoperative appointment with your surgeon. Below are options for such a schedule:
| Medicine | Usual dose | Loading dose |
| sildenafil citrate (Viagra®) |
|
|
| sildenafil citrate (generic medicine) |
|
|
| tadalafil (Cialis®) 20 mg tablets |
|
|
| tadalafil (Cialis) 5 mg tablets |
|
|
Loading dose information
- When you take a loading dose, do so on an empty stomach. Take the medicine about 2 hours before dinner.
- The medicine begins to act after 30–60 minutes. It will remain in the body for up to 8 hours. At any time during these 8 hours, try to achieve sexual arousal from contact with a partner or from self-stimulation. Write down what happened and tell your doctor about it at your next appointment.
- If you do not have any reaction after taking the loading dose for 4 weeks, call your doctor. They can refer you to our sexual health specialists.
to come back to the beginning