Every organ in our body is important, every organ is needed. But special attention must be paid to our “motor” - the heart. Diseases of the cardiovascular system today are one of the main causes of death in all countries of the world. Early diagnosis, disease prevention and treatment of pathologies of the cardiovascular system can not only save your health, but also your life. If the indications are clear and the doctor prescribes medication, then most likely it will be Mildronate.
Mildronate is used during sports
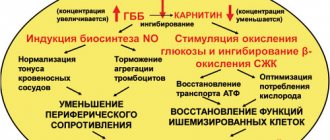
The active ingredient of the drug is meldonium. It is quite successfully and widely used in combination therapy regimens for stroke, cerebral blood supply, ischemia: angina pectoris, myocardial infarction. Moreover, in case of acute damage to the heart muscle, meldonium slows down the growth of necrotic tissue, reducing the time for myocardial rehabilitation. The drug works in the same way for ischemia of brain tissue. It optimizes microcirculation in favor of the tissue area most affected by ischemia. In addition, Mildronate alleviates the consequences of withdrawal syndrome that occurs during the treatment of alcohol dependence.
Also, Mildronate is used in the treatment of vascular pathologies of the eyeball, leading to eye dystrophy.
As a support, the drug is applicable during periods of significant physical activity, as it expands the body's tolerance to it and ensures the flow of oxygen to the tissues. Thus, drugs containing meldonium were taken by athletes during the competitive and out-of-competition period. However, after the introduction of a ban on the use of the drug by the World Anti-Doping Agency (WADA) in 2021, the drug is prohibited for use by athletes.
Mildronate is a metabolic drug that is a synthetic analogue of gamma-butyrobetaine. This substance is found in every cell of the human body. In turn, the drug improves metabolism and energy supply to cells and tissues.
Mildronate is used as:
- cardioprotective;
- antihypoxic (increases resistance to oxygen starvation);
- angioprotective (has a beneficial effect on the vascular wall and microcirculation);
- antianginal agent (directed against angina attacks).
Thus, Mildronate helps improve performance, reduces the severity of manifestations of mental, intellectual and physical stress, and activates tissue and humoral immunity.
Indications
All dosage forms (capsules, tablets, injections) of Mildronate are intended for the treatment of adult patients. A doctor may prescribe the drug in the following cases:
- IHD (in combination with other drugs and treatment methods);
- peripheral arterial disease;
- reduced performance;
- encephalopathy;
- physical overload (including in sports);
- postoperative period (to speed up the recovery of the body);
- chronic heart failure;
- cardialgia (pain in the left side of the chest) caused by dishormonal myocardiopathy;
- bronchial asthma;
- COPD;
- alcohol withdrawal (as an addition to specific therapy);
- stroke.
The drug may be prescribed for reduced performance
Additional indications for the use of drug injections:
- hemorrhage into the vitreous cavity of the eye (hemophthalmos);
- hemorrhage in the retina of the eye;
- thrombosis and occlusion of the central retinal vein or its branches;
- retinopathy of various origins (for example, hemorrhagic or diabetic).
Contraindications
Children under 18 years of age are contraindicated to take Mildronate.
Contraindications to the use of the drug Mildronate (for all forms of release of the drug) are:
- increased individual sensitivity to the active substance of the drug - meldonium - or any of the auxiliary components of the drug;
- intracranial hypertension, including those caused by intracranial tumors and disorders of venous outflow;
- children under 18 years of age;
- pregnancy and breastfeeding period.
Side effects
The drug Mildronate, like almost any drug, has adverse reactions. Following the instructions for use, the following can be distinguished:
- dyspeptic symptoms, manifested by belching, attacks of nausea, vomiting, heartburn, flatulence, a feeling of fullness in the stomach even after a small portion of food;
- allergic reactions (redness, skin rashes, itching and angioedema);
- tachycardia;
- psychomotor overexcitement;
- eosinophilia;
- general weakness;
- changes in blood pressure.
Interaction with other drugs
Mildronate is on the list of main drugs for the treatment of many diseases and goes well with drugs from different groups. Caution is required in the following cases:
- It should not be used with other medicines containing meldronnium.
- To prevent a significant decrease in blood pressure and tachycardia, it should not be used simultaneously with nitroglycerin, alpha-blockers, antihypertensive drugs (especially nifedipine).
- When used together, it enhances the effect of cardiac glycosides and antihypertensive drugs, dilating the coronary vessels.
It can be used together with a large group of drugs for complex treatment: long-acting nitrates, anticoagulants, antiaggregation, diuretics, antiarrhythmics, bronchodilators.
How to take Mildronate
The treatment regimen should be determined by the attending physician, taking into account the severity of the disease and the patient’s medical history. Self-medication can only worsen the condition.
Before taking Mildronate, be sure to consult your doctor!
The drug is taken at 500-1000 mg per day. The appointment can be one-time or divided into appointments throughout the day. It is worth noting that Mildronate can provoke overexcitation, so it is recommended to take it in the first half of the day and no later than 17:00. As a rule, the therapeutic course lasts from 4 to 6 weeks.
The tablets should be taken whole, without chewing or crushing in any other way. Injections are given intravenously, parabulbarly or intramuscularly. Moreover, Mildronate is administered intravenously separately from other medications. It does not need to be diluted with sodium solution. It is not recommended to administer Mildronate intramuscularly, as pain may occur or an allergic reaction may develop.
| Disease | Course duration | Dosage |
| Ischemia (angina pectoris, myocardial infarction), chronic heart failure | 4-6 weeks | As part of complex therapy 0.5-1 g 1-2 times/day |
| Cardialgia developing against the background of dyshormonal myocardial dystrophy | 12 days | 250 mg 2 times/day |
| Cerebrovascular insufficiency | 4-6 weeks | As part of complex therapy 0.5-1 g 1-2 times/day |
| Chronic cerebrovascular accidents | 2-3 times a year for 4-6 weeks | As part of complex therapy 500 mg/day |
| Physical and mental stress and decreased performance | 10-14 days | 500 mg 2 times/day |
| Athletes before training | 14-21 days during the preparatory period and 10-14 days during competitions | 0.5-1 g 2 times/day |
| Chronic alcoholism and withdrawal syndrome | 7-10 days | As part of complex therapy 0.5 g 4 times a day |
| After a heart attack | 4-5 weeks | On the first day, 0.5-1 g of solution is administered intravenously. Then the patient is transferred to tablets - 250 mg 2 times / day |
| Bronchial asthma | 3 weeks | As part of complex therapy 1 time/day |
| Vascular pathologies of the fundus | 10 days | Retrobulbar or subconjunctival 0.5 ml |
| Coronary syndrome | As prescribed by a doctor | IV 0.5-1 g solution 1 time/day. |
It is worth noting that Mildronate is combined with the following medications:
- diuretics;
- bronchodilators;
- antiplatelet agents;
- antiarrhythmic drugs;
- antianginal drugs.
In some cases, Mildronate, due to increased action and the possible development of moderate tachycardia and a decrease in blood pressure, should use the following medications with caution:
- cardiac glycosides;
- beta blockers;
- drugs that lower blood pressure.
As for taking Mildronate in combination with alcohol, in general there is no direct prohibition. However, the combination of the drug and alcoholic beverages can provoke:
- tachycardia;
- pronounced allergic reactions;
- sharp fluctuations in blood pressure;
- dyspeptic symptoms.
The increased risk of various types of complications and the likelihood of relapse of the disease indicate that alcohol should be excluded for the entire period of treatment.
Material and methods
The open study included 119 patients with ischemic stroke (IS) and CCI (in Russian terminology DE), who received care during the period from February 1 to December 31, 2011. The age of the patients varied: with IS - from 29 to 82 years (average - 63.4 years), with DE - from 42 to 85 years (average - 57.7 years). The main vascular disease in the examined patients was hypertension. The duration of increased blood pressure in patients with IS was on average 12.7 years, in patients with DE - 11.9 years.
The patients were divided into two groups. Group 1 consisted of 32 patients with IS (15 men and 17 women). Group 2 consisted of 87 patients with DE (21 men and 66 women).
The criteria for inclusion of patients in the study were: for IS - the presence of a CT-verified stroke at the hospital stage and/or a stroke diagnosed by a neurologist upon discharge from the hospital; for DE – absence of severe cognitive impairment.
Exclusion criteria: for IS - patients staying in hospital for a short period (up to 7 days) and refusal of further treatment; transient ischemic attacks (TIA), the presence of space-occupying brain lesions confirmed by instrumental examination methods, encephalopathy; for DE - the presence of decompensated somatic pathology, severe dementia, Alzheimer's disease, Parkinson's disease, and mental disorders.
65 patients were diagnosed with DE without previous strokes and 22 patients with a history of IS. In case of CCI, the symptom complex was represented by both subjective and objective manifestations of the disease, when 2 or more characteristic symptoms were often repeated and/or existed for at least the last 3 months.
Written consent was obtained from each patient for examination and treatment.
To conduct the study, a protocol was developed that included obtaining information from the anamnesis about the patients’ complaints, objective symptoms, blood pressure (usual for the patient, “office” and after 15 minutes; measured twice using the Korotkoff method on the brachial artery, after which the values of SBP and DBP were recorded) , Heart rate (usual for the patient, during the initial examination and after 15 minutes). Additional examination methods included standard ECG, glucose monitoring (especially for diabetes mellitus), information on taking medications prescribed by the local doctor, subjective data on the condition of patients after treatment (patient complaints), clinical symptoms after treatment and a doctor’s report (indicating side effects , if any), diagnosis (including concomitant pathology). Data from patients in groups 1 and 2 were entered into an identical protocol and then included in the database of the AFIS computer program.
When analyzing the most important risk factors for stroke and DE, special attention was paid to hypertension, ischemic heart disease and diabetes mellitus (DM).
Mildronate was administered intravenously slowly at a dose of 1000 mg (10 ml of 10% solution), diluted in saline. If before the administration of mildronate during IS the SBP was above 200 mm Hg. and/or DBP above 110 mm Hg, and with DE - SBP above 180 mm Hg. and/or DBP above 100 mm Hg, antihypertensive drugs were used (after administration of mildronate). The patient with IS was monitored until he was transferred to the hospital neurologist.
To assess the safety of mildronate, in both groups 1 and 2, all undesirable effects were recorded based on patient complaints and targeted questioning.
All patients with DE (group 2) underwent outpatient treatment. In this case, repeated calls to patients were monitored within 10 days from the date of the initial examination (especially in the first 7 days).
Patients of group 1 (II) were hospitalized in city hospital No. 1. The examination of these patients was carried out at the hospital stage. It consisted of assessing subjective data, neurological deficit, blood pressure upon admission, verification of stroke using CT, ultrasound diagnostics of blood vessels in the brain and neck, determination of glucose and total cholesterol in the blood. The number of hospital days spent, the condition upon discharge from the hospital, the number of discharged patients, deaths, and the number of patients sent for follow-up treatment to an outpatient clinic were also taken into account.
In addition, an analysis of objective symptoms was carried out during the initial examination (by a doctor at an emergency medical service station) and upon admission to the hospital (by a neurologist) with an assessment of the neurological status (analysis of the quality of diagnosis by doctors at an emergency medical service station).
The results of treatment effectiveness were based on subjective data (headache, dizziness, noise in the head, poor memory, emotional lability) and assessment of neurological deficits (motor, vestibulo-cerebellar, sensory and speech disorders). The treatment effect was assessed by the doctor and the patient in both groups as: “improvement” and/or “no change”.
Statistical analysis was carried out using the protocol on the use of mildronate at the prehospital stage, the call card (form 110/y) and the medical history at the hospital stage (form 003/y).
Computer statistical processing of the results was carried out in the automated AFIS system.
Analogs
Analogs of Mildronate: Vasopro, Vazonat, Metamax, Metonat, Trizipine, Mildrakor, Mildrocard, Cardionat, Melfor, Idrinol, Riboxin, Meldonium.
Mildronate is often compared to Cardionate. The fact is that these drugs are based on one active substance, so both drugs have a similar mechanism of action. But they differ in price and release form. You can find Cardionate in the pharmacy only in the form of 250 mg capsules and 500 mg/5 ml injection solution.
Analogs
Also, in the list of the most common replacements for Mildronate tablets with similar drugs is Riboxin. The drug is a means of metabolic action, that is, it participates in biochemical reactions. It has a positive effect on metabolic processes in the heart muscle and improves coronary circulation. Mildronate has a similar effect, but is not itself involved in the synthesis of other substances. Therefore, the effect of Mildronate lasts longer, and the body requires much less of it than Riboxin. As a result, it turns out that the combination of drugs will be optimal.
Important! The decision on combination therapy or replacement of Mildronate with an analogue is made by the attending physician.
Composition and release form
The name of the active substance is meldonium. Mildronate is available in several forms to make it easier to use.
Capsules
This form has two doses - 250 and 500 milligrams of meldonium dihydrate. The capsule shell is white, dense, and inside there is fine powder in the form of white crystals. Additional ingredients: starch, calcium stearate, silicon dioxide. Capsule material: gelatin.
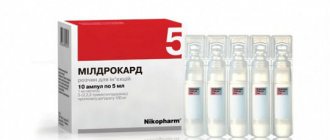
They are in blisters, packed in boxes of 40, 60 capsules.
Solution
Mildronate is available in ampoules for parenteral administration. The ampoule size is 5 milliliters. The volume of meldronnium is 100 milligrams per gram. Packaged in boxes of 10 pcs.
Mildronate syrup (250 milligrams) and Mildronate GX tablets (500 milligrams) are also produced.