pharmachologic effect
Manufacturer: Ozone, Canon Pharma, Russia/Takeda, Denmark
Release form: tablets
Active ingredient: Warfarin
Synonyms: Warfarex, Warfarin Nycomed
Warfarin has an anticoagulant effect due to blocking the synthesis of blood coagulation factors dependent on vitamin K, reducing their content in the blood and slowing down coagulation.
The onset of action usually occurs on the second day of administration, the maximum effect occurs after about a week. After discontinuation of the vitamin-K medication, dependent factors are restored on average within five days.
Drugs with similar effects
The choice of a Warfarin substitute requires medical supervision, examination of the patient's medical history and constant monitoring of INR values. It is important to take into account contraindications, side effects, correctly evaluate the composition and calculate the dosage.
Warfarex
The anticoagulant is intended for long-term use and is indicated for deep vein thrombosis, pulmonary embolism, myocardial infarction, atrial fibrillation, and heart valve replacement. Tablets are taken once a day at an initial dose of 2.5-5 mg. Description:
- Active ingredient: warfarin.
- Mechanism of operation: prevents blood clotting, inhibits the synthesis of factors involved in this process, prevents the formation of blood clots and the increase in existing ones.
- Side effects compared to the analogue: dark red color of the toes, itching, dermatitis, urticaria, nausea, vomiting, jaundice, fever, weakness, alopecia.
- Contraindications: internal bleeding, jaundice, bacterial endocarditis, diabetes mellitus, hemorrhagic diathesis, thrombocytopenia, alcoholism, hypertension, childhood, pregnancy, lactation, gastric ulcer.
- Price, rubles: 125 for 100 tablets.
Article on the topic: Alicaps - instructions for use, indications, composition, release form, mechanism of action and price
Marevan
The indirect-acting anticoagulant has an effect already on the 2-7th day of administration and accumulation in plasma. Its indications for use are deep vein thrombosis, prevention of myocardial infarction and thromboembolism, treatment of transient ischemic attacks and strokes. The starting dose of Marevan is 10.5 mg. Description:
- Active ingredient: warfarin sodium.
- Mechanism of operation: blocks the synthesis of vitamin K-dependent blood clotting factors in the liver.
- Side effects compared to the analogue: bleeding, nausea, vomiting, hepatitis, diarrhea, priapism, vasculitis, skin necrosis, tracheal calcification.
- Contraindications: von Willebrand disease, hemophilia, thrombocytopenia, hemorrhagic diathesis, jaundice, infective endocarditis, exudative pericarditis, diabetes mellitus, exacerbation of gastric ulcers, diverticulosis, malignant tumors, dementia, psychosis, alcoholism, 1st and 3rd trimesters of pregnancy.
- Price, rubles: 150 per 100 pcs.
Clopidogrel
A specific active inhibitor of platelet aggregation has a coronary dilation effect. Inhibition of blood cell aggregation is observed within 2 hours after administration. The drug is indicated for the prevention of thrombotic complications. Clopidogrel is taken orally, regardless of food, 75 mg per day for 1–24 weeks. Description:
- Active ingredient: clopidogrel.
- Mechanism of operation: reduces the binding of adenosine triphosphate to platelet receptors, weakens their aggregation.
- Side effects compared to the analogue: purpura, hematomas, leukopenia, agranulocytosis, hallucinations, paresthesia, vasculitis, hypotension, bronchospasm, diarrhea, dyspepsia, gastritis, colitis, arthralgia, glomerulonephritis, eczema, urticaria.
- Contraindications: severe liver failure, acute bleeding, pregnancy, breastfeeding, age under 18 years.
- Price, rubles: 465 for 14 tablets.
Sinkumar
The indirect anticoagulant contains an alkaloid, the peak of action of which occurs on the 1st–2nd day after administration. Sinkumar is indicated for the treatment and prevention of thrombosis and embolic strokes. The dose is prescribed individually, on the first day it is 4–8 tablets, the daily maintenance dose is 1–6 mg. Description:
- Active ingredient: acenocoumarol.
- Mechanism of action: antagonistic effect on vitamin K, disrupts the synthesis of prothrombin and blood clotting factors.
- Side effects compared to the analogue: nausea, diarrhea, headache, rash, bleeding.
- Contraindications: hemorrhagic diathesis, liver and kidney dysfunction, malignant neoplasms, physical exhaustion, hypovitaminosis K and C, diabetic retinopathy, pregnancy, lactation.
- Price, rubles: 775 for 50 tablets.
Lopirel
The antiplatelet drug has a coronary dilation effect, which manifests itself 2 hours after administration. Lopirel is used for the prevention of atherothrombosis after myocardial infarction or ischemic stroke. The medicine is taken regardless of food, 75 mg once a day. Course – from 7 days to six months. Description:
- Active ingredient: clopidogrel.
- Mechanism of operation: suppresses platelet aggregation, prevents the development of atherothrombosis.
- Side effects compared to the analogue: headache, confusion, diarrhea, colitis, hepatitis, leukopenia, anemia, purpura, hemarthrosis, lichen planus, bullous rash, urticaria, vasculitis, bronchospasm, arthritis, glomerulonephritis, fever.
- Contraindications: liver failure, hemorrhagic syndrome, acute bleeding, pregnancy, lactation, age under 18 years, lactase deficiency.
- Price, rubles: 270 for 14 tablets.
Warfarin - instructions for use
Doses of Warfarin are calculated experimentally. Depending on laboratory parameters. Before prescribing therapy, the patient is measured with prothrombin time (PTT) - this is the time during which a blood clot forms in response to damage to the vessel; normally it is about 11-16 seconds. Then the international normalized ratio (INR) is calculated - equal to the ratio of the patient's PTV to the PTV of a healthy person. Normally, the INR is 0.85–1.35.
In the first four days of treatment, the patient is given 2 Warfarin tablets per day - once, at the same time. On the fifth day, the INR is determined again, which should increase to 2–3 during treatment, and 2.5–3.5 during heart valve replacement and acute myocardial infarction with complications.
Depending on the indicator, a maintenance dose is prescribed. Usually this is 1-3 tablets per day. INR monitoring is carried out every month, and the dose is adjusted if necessary.
The course of treatment with Warfarin depends on the type of disease and individual characteristics of the body and is determined by the doctor. If necessary, the drug is discontinued immediately.
If a patient is re-prescribed Warfarin, then therapy is started with twice the average dose of this particular patient in the first 2 days, then treatment is continued with the average dose. On the fifth day, the INR is also monitored and, if necessary, the dose is adjusted.
For children, the dose is selected based on body weight, functional state of the liver and INR, the level of which should be the same as in adults. The child is treated under the supervision of an experienced pediatrician. In the instructions for use of Warfarin, doses for children are described in more detail.
How to take Warfarin - before or after meals
It does not matter how you take Warfarin, before or after meals. The main thing is to take it at the same time of day, never missing a dose.
Current issues of warfarin therapy for practicing physicians
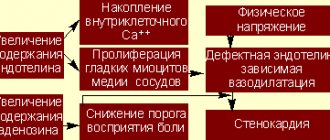
Currently, the effectiveness of Warfarin has been proven for the prevention of thromboembolic complications in patients with atrial fibrillation (AF), after heart valve replacement, in the treatment and prevention of venous thrombosis, as well as in the secondary prevention of cardiovascular episodes in patients who have suffered acute coronary syndrome [1,2 ]. Use of Warfarin for atrial fibrillation The main cause of death and disability in patients with AF without damage to the heart valves is considered ischemic stroke (IS), which is cardioembolic in its mechanism [3–6]. The source of thrombotic masses is in most cases thrombosis of the left atrial appendage, less often - the cavity of the left atrium. Cardioembolic strokes in patients with AF are characterized by extensive cerebral infarction, leading to severe neurological deficits, which in most cases entails permanent disability of the patient [7]. According to large studies, the risk of stroke in patients with AF increases 6 times compared to those with sinus rhythm; it is comparable in paroxysmal and permanent forms of AF and does not depend on the success of antiarrhythmic therapy [3–6,8,9]. The reduction in the risk of IS during warfarin therapy in patients with AF without valvular heart disease has been proven by large randomized studies; it is 61% [10–14]. The determining factor in the choice of antithrombotic therapy tactics in each individual patient with AF is the presence of risk factors (RFs) for thromboembolic complications (TEC). The basis for stratifying a patient with AF is the CHADS2 score, first published in 2001 and retained as the initial risk assessment in the updated guidelines in 2011 [1]. Factors such as chronic heart failure (CHF), arterial hypertension (HTN), age ≥ 75 years and diabetes mellitus are scored 1 point, and IS/TIA or a history of systemic embolism – 2 points. The risk is assessed as high if there are 2 or more points. In 2009, a group of researchers from Birmingham led by G. Lip [15] proposed a new system for stratifying patients, which they called CHA2DS2–VASc. The basis was a 1-year follow-up of a cohort of 1577 patients with AF without heart valve disease who did not receive anticoagulant therapy. The CHA2DS2–VASc scale divides factors into “major” and “clinically associated minor.” “Major” includes previous IS/TIA/systemic embolism and age ≥ 75 years (estimated at 2 points), and “clinically associated minor” includes CHF or asymptomatic decrease in left ventricular ejection fraction ≤40%, hypertension, diabetes mellitus, age 65–74 years, female gender and vascular diseases (myocardial infarction, atherosclerosis of peripheral arteries, atherosclerosis of the aorta), scored 1 point. The fundamental changes are the assessment of female gender and vascular diseases as risk factors and the division of age into two gradations (Table 1). The CHADS2 scale is recommended for the initial determination of the risk of VTE in patients with MA. For patients with a CHADS2 score of ≥2, long-term VKA therapy is indicated under the control of an INR of 2.0–3.0, unless there are contraindications. For a more detailed and thorough risk calculation (in patients with a score of 0–1 on the CHADS2 scale), it is recommended to evaluate the presence of “large” and “clinically associated small” risk factors. Patients with 1 “major” or ≥2 “clinically associated small” risk factors are at high risk of TEC and are recommended to receive VKA therapy unless contraindicated. Patients who have 1 “clinically associated minor” risk factor have an average risk of TEC and are recommended to be treated with VKA or acetylsalicylic acid (ASA) at a dose of 75–325 mg per day. Patients who do not have RF or are at low risk may be prescribed ASA 75–325 mg, or they do not need any antithrombotic therapy. In addition to patients with chronic atrial fibrillation, anticoagulants are required for patients who are planning to restore sinus rhythm. The risk of systemic thromboembolism during cardioversion without the use of anticoagulants reaches 5%, and the use of 4-week warfarin therapy before and after cardioversion can reduce this risk to 0.5–0.8% [16–17]. All patients with a paroxysm of AF lasting 48 hours or more, or when the duration of the paroxysm cannot be determined, are indicated for VKA therapy with maintenance of an INR of 2.0–3.0 for three weeks before and four weeks after cardioversion, regardless of the method of restoring sinus rhythm (electrical or pharmacological). The exclusion of thrombosis of the appendage and cavity of the left atrium according to the emergency echocardiography data allows us to approximate the timing of cardioversion and restore sinus rhythm after achieving the target INR range of 2.0–3.0. However, in this case, the patient is advised to take Warfarin therapy for 4 weeks to exclude normalization thromboembolism. after cardioversion. When performing urgent cardioversion, heparin therapy (unfractionated or low molecular weight heparin) is indicated. If the AF paroxysm lasted 48 hours or more or when it is impossible to determine the duration of the paroxysm, after emergency cardioversion, VKA therapy is indicated for 4 weeks. If the duration of the paroxysm did not exceed 48 hours in a patient who does not have FR TEO, it is possible to perform cardioversion after the administration of heparin without the subsequent administration of Warfarin. In patients with risk factors for stroke or a high probability of recurrent AF, VKA therapy is indicated indefinitely, regardless of maintenance of sinus rhythm immediately after cardioversion. Approaches to anticoagulation therapy for cardioversion performed in connection with atrial flutter are similar to those used for atrial fibrillation [1–2]. Warfarin in patients with artificial heart valves The main danger to the life of patients with artificial heart valves is thromboembolic complications, the source of which are blood clots that form on the surface of the valve prosthesis. The risk of prosthetic valve thrombosis, a life-threatening complication, in the absence of VKA therapy reaches 8–22% per year [2,18]. Prescribing Warfarin reduces the risk of thromboembolism by 75%, therefore, when installing mechanical prosthetic heart valves, VKAs are mandatory and cannot be replaced by ASA. The exception is patients with bioprostheses without FR TEO, the duration of VKA therapy for whom is three months; in all other cases, treatment should be lifelong. Risk factors for patients with artificial heart valves are a history of thromboembolism, AF, circulatory failure, and atriomegaly. The level of anticoagulation in the vast majority of cases should be within the INR range of 2.5–3.5. The exception is patients after implantation of a modern bicuspid aortic valve prosthesis in the absence of other risk factors for thromboembolism, in this case the target INR range is 2.0–3.0 [2.18]. Indications for VKA therapy in patients after heart valve replacement are presented in Table 2. Warfarin in the treatment of venous thrombosis The duration of warfarin therapy in patients after deep vein thrombosis (DVT) or pulmonary embolism (PETA) associated with a reversible factor is 3 months. The duration of warfarin therapy in patients after unprovoked DVT/TEOLA is at least 3 months. In the future, it is necessary to evaluate the risk-benefit ratio of continuing VKA therapy. Long-term (lifelong) use of VKAs is recommended for patients who have experienced a first episode of unprovoked proximal DVT/TEOLA, have adequate INR monitoring, and do not have bleeding risk factors. Long-term VKA therapy is indicated for patients who have experienced a second episode of unprovoked venous thrombosis. The principles of treatment for asymptomatic and symptomatic venous thrombosis are similar. The level of anticoagulation for the prevention of recurrent venous thrombosis corresponds to an INR of 2.0–3.0 [2]. VKA in the secondary prevention of coronary heart disease The effectiveness of Warfarin in the secondary prevention of coronary artery disease was studied in the ASPECT-2, APRICOT-2, WARIS-II, CHAMP studies [19–22]. These studies differed in design, anticoagulation regimens, the presence of concomitant ASA therapy and the dose of the latter. The effectiveness of the combination of Warfarin and ASA was higher than ASA monotherapy, but the risk of hemorrhagic complications was higher in the combination therapy group. In this regard, in routine clinical practice, without special indications, Warfarin is not prescribed to patients with coronary artery disease. Practical aspects of VKA therapy Warfarin therapy should be selected based on a dose titration schedule to achieve target INR values. Before prescribing Warfarin, it is necessary to assess the presence of contraindications, the risk of bleeding in the patient, and also conduct an examination aimed at verifying potential sources of bleeding. Absolute contraindications to the use of Warfarin are an allergy to the drug, a history of hemorrhagic stroke, active bleeding, and significant thrombocytopenia. All other conditions are relative contraindications, and the choice is made based on the individual balance of benefit and risk of bleeding. Before prescribing Warfarin, it is necessary to clarify whether the patient has a history of hemorrhagic complications and to conduct an examination aimed at clarifying the status of potential sources of bleeding. The plan for mandatory and additional examination is presented in Figure 1. It is necessary to assess the risk of bleeding in all patients before prescribing antithrombotic therapy, taking into account the comparable risk of ASA and Warfarin, especially in elderly patients. In 2010, experts from the European Society of Cardiology introduced the HAS-BLED scale, which allows one to calculate the risk of bleeding in a patient. The risk is assessed as high if there are ≥3 points, however, this is not a contraindication for anticoagulant therapy, but regular monitoring is required during VKA or ASA therapy (Table 3). As a starting dose of Warfarin, it is advisable to use 5–7.5 mg during the first two days with further titration of the dose, focusing on the achieved INR level (Fig. 2). Lower starting doses of Warfarin (5 mg or less) are recommended for patients over 70 years of age, with low body weight, CHF or renal failure, as well as for patients with underlying liver dysfunction, concomitant use of amiodarone, and patients who have recently undergone surgery. The American recommendations of 2012 [2] indicate the starting dose of Warfarin (10 mg), however, taking into account the difference between the American population and the Russian population, as well as the increased risk of bleeding during the saturation period, it is advisable not to exceed the initial starting dose 7. 5 mg. In addition, immediately prescribing high starting doses of Warfarin (10 mg or more) is not recommended, as this leads to a decrease in the level of the natural anticoagulant protein C, which can lead to the development of venous thrombosis. During dose selection, INR monitoring is carried out once every 2–3 days. After receiving the INR results within the target range, the dose of Warfarin twice is considered selected, and in the future, INR monitoring is carried out once a month. The target INR range for patients with AF without heart valve damage and after venous thrombosis when using Warfarin without antiplatelet agents is 2.0–3.0, when combined with antiplatelet agents – 2.0–2.5. In patients after implantation of artificial heart valves, in most cases the target INR is 2.5–3.5. Currently, polymorphisms have been identified in the main biotransformation gene of Warfarin - CYP2C9 and the target molecule of its action - VKORC1. Carriers of mutant alleles require a lower maintenance dose of Warfarin, while the frequency of bleeding and episodes of excessive hypocoagulation is higher. Currently, there are algorithms for calculating the dose of Warfarin based on genotyping [23–28], the implementation of which is quite possible both from the point of view of routine practice and from an economic point of view. However, the recommendations [1–2] state that in the current absence of data from specific randomized trials, the use of a pharmacogenetic approach to VKA prescription for all patients is not recommended. Warfarin is a drug that is characterized by interindividual differences in drug response due to a number of factors, both external (diet, drug interactions) and internal (the patient's physical condition, age), as well as genetically determined. To exclude unwanted drug interactions when prescribing concomitant therapy, preference should be given to drugs whose effect on the anticoagulant effect of Warfarin is insignificant (Fig. 3). The use of drugs that affect the metabolism of VKA requires monitoring the INR after 3–5 days and, if necessary, adjusting the dose of Warfarin. Patients taking anticoagulants require a patronage system, which is due to the need for regular monitoring of INR, drug dose adjustment and assessment of other factors affecting INR values. It is advisable to give the patient a reminder. Fluctuations in INR values can be caused by several factors: 1. Laboratory error. 2. Significant changes in dietary vitamin K intake. 3. The influence of changes in somatic status, taking medications, alcohol and substances of plant origin on the metabolism of Warfarin. 4. Lack of adherence to treatment with Warfarin. To exclude food interactions, patients taking Warfarin should be advised to adhere to the same diet, limit alcohol consumption, and not take medications and herbal substances on their own without consulting a doctor, taking into account the possibility of their influence on the metabolism of Warfarin. INR values from measurement to measurement in the same patient may vary within the therapeutic range. Fluctuations in INR that are slightly outside the therapeutic range (1.9–3.2) are not grounds for changing the dose of the drug. It is necessary to monitor the INR value after 1 week, after which, if necessary, adjust the dose of Warfarin. To avoid significant fluctuations in the level of anticoagulation, it is advisable to reduce the dosage of Warfarin at INR values of more than 3.0 but less than 4.0, without skipping the next dose of the drug. There is no average daily dose of Warfarin. The dose should be adjusted based on the target range. The question of what is considered true resistance to Warfarin remains open to this day. It may be worth talking about true resistance if the prescription of a dose of Warfarin exceeding 20 mg per day did not lead to the achievement of a therapeutic level of anticoagulation. This is the so-called “pharmacodynamic (or true) resistance”, which can be confirmed by identifying a high concentration of Warfarin in the blood plasma in the absence of an increase in INR values. The number of such cases among patients, according to specialized studies, does not exceed 1% [27,28]. Risk of bleeding during VKA therapy The development of hemorrhagic complications is the most dangerous side effect of VKA therapy and the main reason for non-prescription of drugs in this group. The incidence of major bleeding during warfarin therapy is about 2%, and fatal bleeding is about 0.1% per year [3–7,29–32]. Non-hemorrhagic side effects of Warfarin are very rare - allergic reactions (itching, rash), gastrointestinal disorders (nausea, vomiting, abdominal pain), transient baldness. The main risk factors for hemorrhagic complications are the degree of hypocoagulation, advanced age, interactions with other drugs and invasive interventions, and initiation of therapy [29–32]. To improve the safety of therapy, it is necessary to identify contraindications and potential sources of bleeding, take into account concomitant pathology (CHF, chronic renal failure, liver failure, postoperative period) and therapy. The occurrence of major bleeding (i.e., leading to death, cardiac/respiratory problems, other irreversible consequences, requiring surgical treatment or blood transfusion) always requires urgent hospitalization of the patient to find its cause and quickly stop it. Resumption of warfarin therapy after major bleeding is possible only if the cause of the bleeding is found and eliminated. The target INR range should be reduced to 2.0–2.5. The occurrence of minor hemorrhagic complications (any internal or external bleeding that does not require hospitalization, additional examination and treatment) requires temporary discontinuation of Warfarin until the bleeding stops, a search for its possible cause and dose adjustment of Warfarin. If minor bleeding occurs against the background of an INR value > 4.0, it is necessary to find out the possible reasons for the development of excessive hypocoagulation (primarily taking medications that affect the metabolism of VKA). Warfarin therapy can be resumed after stopping minor bleeding if the INR is <3.0. In case of recurrence of small hemorrhages, the target INR level should be reduced to 2.0–2.5. Excessive anticoagulation is a predictor of bleeding, so any, even asymptomatic, increase in INR levels above the therapeutic range requires the attention of a doctor. It is necessary to clarify with the patient the possible reasons for the increase in INR (primarily drug interactions, as well as such reasons for the development of excessive hypocoagulation as CHF, liver failure, hyperthyroidism, alcohol consumption). Detection of an asymptomatic increase in INR in a patient in the absence of any immediate need for invasive intervention requires temporary withdrawal of Warfarin with subsequent adjustment of its dose, but there is no need to administer fresh frozen plasma or prothrombin complex concentrate. Vitamin K promotes de novo synthesis of vitamin K-dependent coagulation factors due to its influence on carboxylation processes, so the effect after its intake occurs slowly and it is useless for the rapid restoration of vitamin K-dependent coagulation factors. The domestic drug phytomenadione available to doctors in capsules of 0.1 g, containing a 10% solution of vitamin K1 in oil, cannot be used to reduce the level of INR, since a dose of vitamin K equal to 10 mg causes resistance to the action of VKA for 7–10 days. The risk of bleeding increases during invasive interventions and surgical operations. The basis for correctly selected perioperative tactics in a patient taking Warfarin is an assessment of the risk of bleeding and thromboembolic complications (Fig. 4). In 2010, ESC guidelines [1] recommend early resumption of anticoagulant therapy in patients at high risk of thromboembolic complications, provided there is adequate hemostasis. ESC experts also published an addition to the existing recommendations [33] that there is no need to discontinue warfarin in patients at high risk of stroke during dental extraction, cataract removal and endoscopic removal of polyps from the gastrointestinal tract, provided that modern technology is used and adequate hemostasis is ensured. In this case, in the author’s own opinion, it is advisable to skip taking Warfarin on the eve of the intervention, provided that adequate hemostasis is maintained. Currently, there are portable devices for measuring INR levels. A meta-analysis conducted by S. Heneghan in 2006 [34] showed that self-monitoring of INR improves the outcomes of patients receiving warfarin. However, a necessary condition for adequate self-monitoring using a portable device is medical supervision for the correct interpretation of the results of the analysis and correction of factors that influence anticoagulant therapy. Conclusion Currently, Warfarin is the main drug for the prevention of thromboembolic complications in patients with myocardial infarction after heart valve replacement and venous thrombosis. The determining factor in the effectiveness of therapy with vitamin K antagonists is the target INR range, which should be attempted in every patient. The frequency of hemorrhagic complications, as well as the need for constant laboratory monitoring, are the main reasons for non-prescription or discontinuation of Warfarin in real clinical practice. The emergence of new antithrombotic drugs that do not require regular laboratory monitoring also requires doctors to acquire practical clinical experience. Existing algorithms for selecting an individual maintenance dose of Warfarin, a system of patronage and regular laboratory monitoring of INR can improve the safety of anticoagulant therapy.
Literature 1. Guidelines for the management of patients with atrial fibrillation 2011. The task force for the management of patients with atrial fibrillation of European Society of Cardiology. 2. ACCP American college of Chest Physicians Evidence–Based Clinical Practice Guidelines (9th Edition) // Chest. – 2012. in press. 3. Wolf PA, Dawber TR, Thomas E. Jr et al. Epidimiologic assessment of chronic atrial fibrillation and risk of stroke: The Framingham Study // Neurol. – 1978. – Vol. 28. – P. 973–977. 4. Onundarson PT, Thorgeirsson G., Jonmundsson E. et al. Chronic atrial fibrillation – Epidimiologic features and 14 year follow-up: A case control study // Eur. Heart. J. – 1987.– Vol. 3. – P.521–27. 5. Flegel KM, Shipley MJ, Rose G. Risk of stroke in non-rheumatic atrial fibrillation // Lancet. – 1987.– Vol. 1.– P.526–529. 6. Tanaka H., Hayashi M., Date C. et al. Epidemiologic studies of stroke in Shibata, a Japanese provincial city: Preliminary report on risk factors for cerebral infarction // Stroke. – 1985. – Vol. 16.– P. 773–780. 7. Hylek MPH, Alan S. Go, Yuchiao Chang et al. Effect of Intensity of Oral Anticoagulation on Stroke Severity and Mortality in Atrial Fibrillation // NEJM. – 2003. –Vol. 349. – P.1019–1026. 8. Wyse DG, Waldo AL, DiMarco JP et al. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation // NEJM. – 2002. – Vol. 347. – P.1825–1833. 9. Stefan H. Hohnloser, Karl–Heinz Kuck, Jurgen Lilienthal. For the PIAF Investigators Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomized trial // Lancet. – 2000. – Vol. 356. –P. 1789 – 1794. 10. Petersen P., Boysen G., Godtfredsen J. et al. Placebo–controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study // Lancet. – 1989. – Vol. 28;1(8631). – P.175–179. 11. Secondary prevention in nonrheumatic atrial fibrillation and transient ischemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group // Lancet. – 1993. – Vol. 342. – P. 1255–1262. 12. Hart RG, Pearce LA, McBride R. et al. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I–III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators // Stroke. – 1999. –Vol. 30(6). – P.1223–1229. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. 13. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators // NEJM. – 1990. – Vol. 323. – P.1505–1511. 14. Ezekowitz MD, Bridgers SL, Javes KE et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation // NEJM. – 1992. – Vol. 327, No. 20. – P. 1406–1413. 15. Lip GYH, Nieuwlaat R., Pisters R. et al. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor–Based Approach The Euro Heart Survey on Atrial Fibrillation // Chest. – 2010.– Vol. 137. – P. 263–272. 16. Arnold AZ, Mick MJ, Mazurek RP Role of prophylactic anti-coagulation for direct cardioversion in patients with atrial fibrillation or atrial flutter // J. Am. Coll. Cardiol.– 1992.– Vol. 19.– P. 851–855. 17. Manning WJ, Silverman DI, Keighley CS et al. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short term anticoagulation: final results of a prospective 4.5–year study // J. Am. Coll. Cardiol. – 1995. – Vol. 25(6). – P.1354–1361. 18. Dzemeshkevich S.L, Panchenko E.P. Anticoagulant therapy in patients with valvular heart disease // RMZh. – 2001. – T. 9, No. 10. – P. 427–430. 19. Anticoagulants in the Secondary Prevention of Events in Coronary Thrombosis (ASPECT) Research Group. Effect of long-term oral anticoagulant treatment on mortality cardiovascular morbidity after myocardial infarction // Lancet. – 1994.– Vol. 343. – P. 499–503. 20. Brouwer MA, van den Bergh PJ, Aengevaeren WR et al. Aspirin plus coumarin vs aspirin alone in the prevention of reocclusion after fibrinolysis for acute myocardial infarction: results of the Antithrombotics in the Prevention of Reocclusion in Coronary Thrombolysis (APRICOT)–2 Trial // Circulation. – 2002. – Vol.106. – P.659–665. 21. Hurlen M., Smith P., Arnesen H. et al. Warfarin–Aspirin Reinfarction Study II WARIS –II // NEJM. – 2002. – Vol. 347. – P.969–974. 22. Fiore LD, Ezekowitz MD, Brophy MT et al. For the Combination Hemotherapy and Mortality Prevention (CHAMP) Study Group Warfarin combined with low dose aspirin in myocardial infarction did not provide clinical benefit beyond that of aspirin alone // Circulation. – 2002. – Vol.105. – P. 557–563. 23. Holbrook AM, Jennifer A. Pereira, Renee Labiris et al. Systematic Overview of Warfarin and Its Drugand Food Interactions // Arch. Intern. Med. – 2005. – Vol. 165. – P. 1095–1106. 24. Rieder MJ, Reiner AP, Gage BF et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose // NEJM. – 2005. –Vol. 352(22). – P.2285–2293. 25. Higashi MK, Veenstra DL, Kondo LM et al. Association between CYP2C9 genetic variants and anticoagulation–related outcomes during warfarin therapy // JAMA. – 2002. –Vol. 287. – P.1690–1698. 26. Tabrizi AR, Zehnbauer BA, Borecki IB et al. The frequency and effects of cytochrome P450 (CYP) 2C9 polymorphisms in patients receiving warfarin // J. Am. Coll. Surg. – 2002. – Vol. 194. – P.267–273. 27. Harrington DJ, Underwood S, Morse C et al. Pharmacodynamic resistance to warfarin associated with a Val66Met substitution in vitamin K epoxide reductase complex subunit 1 // Thromb. Haemost. – 2005. – Vol. 93. – P. 23–26. 28. Bodin L., Horellou MH, Flaujac C. et al. A vitamin K epoxide reductase complex subunit–1 (VKORC1) mutation in a patient with vitamin K antagonist resistance // J. Thromb. Haemost. –2005. – Vol. 3. – P.1533–1535. 29. Fihn SD, McDommel M, Matin D et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group // Ann. Intern. Med. – 1993. – Vol. 118(7). – P. 511–520. 30. Mhairi Copland, Walker ID, Campbell R. et al. Oral Anticoagulation and Hemorrhagic Complications in an Elderly Population With Atrial Fibrillation // Arch. Intern. Med. – 2001. – Vol. 161, No. 17. – P. 24. 31. Levine MN, Raskob G., Landefeld S., Kearon C. Hemorrhagic complication of anticoagulant treatment // Chest. – 2001. – Vol. 19(1 Suppl). – P.108S–121S. 32. Palareti G., Leali N., Cocceri S., Poggi M. et al. Hemorrhagic complications of oral anticoagulant therapy: results of a prospective multicenter study ISCOAT (Italian Study on Complications of Oral Anticoagulant Therapy) // G. Ital. Cardiol. – 1997. – Vol. 27(3).– P.231–243. 33. Skolarus LE, Morgenstern LB, Froehlich JB et al. Guidline–Discordant Perioprocedural Interruption in warfarin therapy // Circ. Cardiovasc. Qual. Outcomes. – 2011. –Vol. 4. 34. Heneghan C., Alonso–Coello P., Garcia–Alamino JM et al. Self-monitoring of oral anticoagulation: a systematic review and meta-analysis // Lancet. – 2006. – Vol. 367. – P. 404–411.
Warfarin analogues that do not require INR control
It is important to decide what to replace Warfarin with so as not to control the INR, since this brings a lot of inconvenience for the patient, frequent visits to the clinic and tests.
Warfarin analogues that do not require INR control include:
- antiplatelet agents - Plavix, Cardiomagnyl, Thrombo ACC, Aspirin Cardio;
- direct-acting anticoagulants - Pradaxa, Eliquis, Xarelto, Clexane.
Prices for Warfarin analogues can be compared in the table
| Analogue | Active substance | Average price of a package for a minimum course of treatment, rub. | Country of origin |
| Warfarin | Warfarin | 60 | Russia |
| Warfarin Nycomed | Warfarin | 100 | Denmark |
| Cardiomagnyl | Acetylsalicylic acid + Magnesium hydroxide | 150 | Germany |
| Thrombo Ass | Acetylsalicylic acid | 80 | Austria |
| Clexane | Enoxaparin Sodium | 2000 | France |
| Pradaxa | Dabigatran etexilate | 1900 | Germany |
| Xarelto | Rivaroxaban | 1700 | Germany |
| Eliquis | Apixaban | 1000 | USA |
Pradaxa
Manufacturer: Boehringer Ingelheim, Germany
Release form: capsules
Active ingredient: Dabigatran etexilate
Warfarin substitute Pradaxa is a German original drug, a direct-acting anticoagulant, which is converted into an active form in the body and directly and reversibly inhibits thrombin activity. Therefore, this analogue prevents the formation of blood clots.
An analogue of Pradaxa is produced in 75, 110 and 150 mg doses, the therapeutic dose is selected by the doctor. Capsules should not be opened.
The Warfarin analog Pradaxa is used for the treatment of acute thrombosis and thromboembolism and significantly reduces the risk of death from these diseases.
An INR test is not required during treatment with Pradaxa.
Sinkumar
Manufacturer: Meda Pharma, Germany
Release form: tablets
Active ingredient: Acenocoumarol
Analogue Sinkumar is an indirect anticoagulant. Like Warfarin, it is a vitamin K antagonist and inhibits the synthesis of vitamin K-dependent blood clotting factors.
The Warfarin analog Sincumar is used to treat pulmonary thromboembolism, coronary artery thrombosis and phlebothrombosis.
Treatment with Sinkumar should be accompanied by constant monitoring of coagulation parameters, namely prothrombin time and INR.
Clexane
Manufacturer: Sanofi, France
Release form: injection solution
Active ingredient: Enoxaparin sodium
Synonyms: Enixum, Anfibra, Gemapaksan
The Clexane analogue is a direct anticoagulant that acts by inhibiting the function of the thrombokinase enzyme, a blood clotting factor.
Clexane is a low molecular weight heparin, an injection solution with various concentrations of the active substance.
The drug is administered subcutaneously or intravenously; it cannot be used intramuscularly. Treatment is often carried out in a hospital setting for patients on bed rest.
No additional monitoring of blood parameters is required.
Comparison with Xarleto
Xarleto is an anticoagulant based on rivaroxaban. It is usually produced in the form of tablets, but there are also injection solutions in ampoules. The following mechanisms of action are distinguished:
- activation of Factor Xa;
- suppression of the conversion of prothrombin to thrombin;
- decreased fibrin thrombus formation, platelet activation.
The effect on the coagulation system depends on the dose of the drug. The higher it is, the stronger the effect. The drug is different in that the doctor does not need to periodically monitor the INR. This indicator is calibrated for the drug.
The drug increases APTT, but the doctor does not need to periodically measure this parameter; this is the main difference between the drug and warfarin-based drugs.
When taking tablets, absorption occurs in the gastrointestinal tract. If you use an injection, the active substance directly enters the bloodstream. The connection with blood plasma proteins is high. Metabolism occurs in the liver and excretion occurs through urine and feces. Both metabolites and stored active components are removed. Indications for use:
- prevention, treatment of stroke, thromboembolism, fibrillation;
- treatment of deep vein thrombosis, prevention of relapses.
Xarleto is recommended by phlebolologists and cardiologists due to good clinical trial results. However, it has a number of negative consequences, which are taken into account when prescribing for patients of a certain age and condition of internal organs.
Side effects and contraindications
Despite the absence of the need to check the coagulation system during treatment, the drug has a number of negative reactions on the body. It is prohibited to be used in combination with other anticoagulants. Bleeding will inevitably occur. It is contraindicated in the following conditions:
- bleeding of external and internal organs;
- peptic ulcer of the stomach and duodenum, malignant tumors, injuries of the brain and spinal cord, recent operations;
- pathologies of the venous system (varicose veins, thrombophlebitis);
- inflammatory liver diseases accompanied by coagulation disorders;
- severe kidney pathologies;
- pregnancy, breastfeeding, minor age.
The drug can be used, but with caution if there is a low risk of bleeding during inflammation of internal organs, mild or moderate kidney damage. It has the following side effects:
- reduction of blood sprouts;
- dizziness, headache;
- hemorrhage, bleeding gums and nasal passages;
- decreased liver and kidney function;
- fever.
If any adverse reactions occur, the drug should be discontinued immediately. Before prescribing therapy, tell the doctor about all the medications the patient is taking to avoid cross-interactions. This is especially true for non-steroidal anti-inflammatory drugs.
Warfarin or Aspirin Cardio – which is better?
Manufacturer: Bayer, Germany
Release form: film-coated tablets
Active ingredient: Acetylsalicylic acid
Synonyms: Thrombo ACC, Thrombopol, CardiASK, ASC Cardio
Warfarin analogue Aspirin Cardio is an original German drug that has an antiplatelet effect by blocking the enzyme cyclooxygenase-1, and thus preventing platelet aggregation.
The analogue of Aspirin Cardio is used mainly for prophylactic purposes to prevent cerebrovascular accidents, stroke, acute myocardial infarction, and complications after vascular surgery.
One of the indications for the use of the Aspirin Cardio analogue is stable and unstable angina.
The active ingredient Acetylsalicylic acid in doses of 500 mg has an analgesic, antipyretic and anti-inflammatory effect.
Characteristics of Pradaksa
Pradaxa is an anticoagulant based on dabigatran etexilate. It is used orally, intravenously. It refers to drugs that inhibit thrombin. The following pharmacological actions are distinguished:
- suppression of thrombin formation;
- inhibition of the transition of fibrinogen to fibrin;
- elimination of platelet aggregation.
The more active substance is in the blood plasma, the higher its effectiveness. The medicine prolongs the APTT, but does not affect the INR. Therefore, coagulation measurements are not required during therapy. This is the main difference from warfarin-based drugs. The product has the following indications for use:
- venous thromboembolism after surgery;
- risk of stroke, atrial fibrillation;
- deep vein thrombosis, pulmonary embolism.
The medicine is strictly prohibited during pregnancy and lactation. During this period, the ratio of clotting factors changes, which can lead to DIC syndrome during childbirth. This is a condition for which therapy with such agents is not recommended.
Side effects and contraindications
Negative consequences arise from individual intolerance. They often form if the patient carries out therapy independently. The following types of side effects are distinguished:
- decrease in red blood cells and platelets;
- local, systemic allergy;
- bleeding, hemoptysis, hemorrhage into internal organs;
- dyspepsia, nausea, inflammation of various parts of the gastrointestinal tract;
- liver dysfunction, jaundice.
There are quite a lot of contraindications. Therefore, before prescribing the drug, doctors collect the patient’s medical history and be sure to identify the drugs that he uses. Contraindications for use:
- increased sensitivity to the product;
- severe diseases of the liver, kidneys, gastrointestinal tract;
- hemorrhagic diathesis, reduced number of platelets and clotting factors, periodic bleeding;
- malignant neoplasms, brain damage, ophthalmological operations, increased intracranial pressure, varicose veins;
- use of any types of anticoagulants;
- minor age, pregnancy, lactation.
There are diseases for which the drug is used, but with caution. Then it is recommended to carry out a detailed coagulogram and constantly calculate the number of platelets and clotting factors. If a side effect occurs, the drug is immediately discontinued.
Warfarin or Thrombo ACC – which is better?
Manufacturer: Lannacher, Austria
Release form: film-coated tablets
Active ingredient: Acetylsalicylic acid
Synonyms: Aspirin Cardio, Thrombopol, CardiASK
To thin the blood, Warfarin can be replaced with the Austrian drug Trombo ACC, which contains acetylsalicylic acid in 50 and 100 mg doses. In these tiny doses it has antiplatelet properties.
An analogue of Thrombo ACC can be freely purchased without a doctor’s prescription and taken 1 tablet daily for the prevention of heart attack, stroke, and other diseases accompanied by increased thrombus formation.
An analogue of Thrombo ACC is also prescribed after vascular surgery.
To prevent the negative effects of acid on the stomach, Trombo ACC tablets are coated with a special enteric coating. Therefore, they should not be chewed or divided in half.
Warfarin or Cardiomagnyl - which is better?
Manufacturer: Takeda, Germany
Release form: film-coated tablets
Active ingredient: Acetylsalicylic acid + Magnesium hydroxide
Synonyms: Fasostabil, TromboMag, Trombital
A substitute for Warfarin without INR control, Cardiomagnyl is an over-the-counter complex drug consisting of acetylsalicylic acid and magnesium hydroxide, which is needed to protect the gastric mucosa from the damaging effects of acid. If acetylsalicylic acid is used for a long time without protecting the stomach, it can lead to ulcers or gastritis.
The antiplatelet drug Cardiomagnyl is used for the prevention of cardiovascular diseases characterized by increased thrombus formation, including stroke or heart attack.
For preventive purposes, the Cardiomagnyl analogue is taken 150 mg on the first day, then 75 mg once a day on an ongoing basis.
If there have already been cases of heart attack or stroke, the drug is prescribed 150 mg daily.
Warfarin or Xarelto - which is better for thrombosis and atrial fibrillation
Manufacturer: Bayer, Germany
Release form: film-coated tablets
Active ingredient: Rivaroxaban
The imported analogue of Xarelto is an original German drug that has no synonyms. This is a direct-acting anticoagulant, selectively suppresses factor Xa, as a result prevents the formation of blood clots and does not require regular blood tests to determine PTT and INR.
Doses of the Xarelto analog range from 2.5 to 15 mg. The doctor decides which one to use for certain indications.
Doses of up to 10 mg of Xarelto are prescribed for prophylaxis once a day, 20–30 mg per day is required for the treatment of thromboembolism, atrial fibrillation, and venous thrombosis.
New generation warfarin analogues
Taking the pill comes with many complications, including constantly measuring the International Normalized Ratio value. New blood thinners have been developed. It is not always possible to take a Warfarin analogue without INR control. The drugs have fewer side effects and are used for treatment and prevention.
Disadvantages: high cost, they are rarely on sale.
Pradaxa
A new generation antithrombotic substitute for Warfarin is used for the prevention of venous thromboembolism, stroke, systemic thromboembolism, and atrial fibrillation. Pradaxa capsules are taken 1 pc. 1-2 times a day, regardless of meals. Description:
- Active ingredient: dabigatran etexilate.
- Mechanism of action: anticoagulant effect, inhibits thrombin activity.
- Side effects in comparison with the analogue: thrombocytopenia, urticaria, bronchospasm, intracranial bleeding, hematomas, hemoptysis, dyspepsia, dysphagia, gastroesophagitis, hyperbilirubinemia, hemarthrosis, hematuria, arrhythmia, hypertension.
- Contraindications: renal failure, active bleeding, hemorrhagic diathesis, liver and kidney dysfunction, age under 18 years.
- Price, rubles: 3175 for 60 capsules.
Xarelto
A direct-acting anticoagulant is indicated for the prevention of venous thromboembolism after major surgery. Xarelto is taken orally, regardless of food intake, 10 mg once a day for a course of 2-5 weeks. Description:
- Active ingredient: rivaroxaban.
- Mechanism of operation: prolongs the time of thrombus formation, protects against internal coronary bleeding.
- Side effects compared to the analogue: anemia, hemorrhagic complications, weakness, dizziness, shortness of breath, angina, hematomas, dyspepsia, headache, allergic dermatitis, fever, jaundice.
- Contraindications: internal bleeding, pregnancy, lactation, age under 18 years, atherosclerosis, cardiovascular failure.
- Price, rubles: 9475 for 100 tablets.
Warfarin or Eliquis – which is better?
Manufacturer: Pfizer, USA
Release form: film-coated tablets
Active ingredient: Apixaban
Eliquis is an analogue of Warfarin that does not require INR control; it is a direct anticoagulant.
The drug blocks factor Xa, which stimulates blood clotting, and prevents blood clots.
The American analogue Eliquis is taken to prevent thrombosis during endoprosthetics, stroke and thrombosis in patients with atrial fibrillation.
The Warfarin analog Eliquis is available in the form of tablets of 2.5 and 5 mg. The usual dosage regimen is 2 tablets per day, 2.5 mg for prevention, and 5–10 mg for the treatment of venous thrombosis and pulmonary embolism.
Reviews from patients indicate that the medication is well tolerated.
Warfarin or Phenilin
, Ukraine
Release form: tablets
Active ingredient: Phenindione
Warfarin analog Phenilin is an indirect anticoagulant that blocks the enzyme K-vitamin reductase. It is used for the treatment and prevention of thrombosis and embolism, for prosthetic heart valves.
Doses are selected based on laboratory tests.
The disadvantage of the Phenilin analogue is the requirement for constant monitoring of not only prothrombin time and INR, but also regular analysis of coagulograms, thromboelastograms and platelet counts.
Characteristics of Warfarin
Warfarin is an indirect anticoagulant. Based on the active ingredient of the same name. The medicine is produced in different forms. It is used orally, intravenously. The latter action is more effective, since the active component minutes physiological barriers, immediately entering the systemic bloodstream.
The synthesis of vitamin K and clotting factors stops, so blood clots stop forming.
Absorption after oral administration occurs in the gastrointestinal tract. The binding of blood plasma proteins is high. Metabolism occurs in the liver, excretion occurs in bile and urine. If you take the medicine for a long time, follow a diet. Nutrition is prescribed by a phlebologist and nutritionist.
The product is indicated for use in the treatment and prevention of the following diseases:
- thrombosis;
- thromboembolism;
- heart attack caused by blockage by a blood clot;
- ischemic attacks, stroke.
The product is used only according to the instructions from the doctor; self-medication should not be carried out. This is due to the fact that it causes many negative reactions, even death, if used incorrectly.
Side effects, contraindications for use
Negative actions occur frequently, especially in patients with individual intolerance. Therefore, before prescribing the drug, the patient is completely examined, conducting a detailed coagulogram. The following side effects are possible:
- increased bleeding;
- dyspepsia, impaired stool formation, discharge of blood in feces;
- inflammation of blood vessels, necrosis of skin areas;
- local and systemic allergic reactions;
- increased liver enzymes, jaundice;
- nose bleed.
The medicine is strictly contraindicated in cases of bleeding, severe kidney and liver diseases, disseminated intravascular coagulation syndrome, and a decrease in platelet count. It is not recommended to use it for inflammatory diseases, damage to veins and arteries. Not compatible with alcohol. Severe defects may occur with gastric and duodenal ulcers, endocarditis, arterial hypertension, a tendency to strokes and intracranial hemorrhages.
INR control
INR (international normalized ratio) is a blood test in a detailed coagulogram, recognized to show the quality of the coagulation system. If the value is above the average acceptable level, there is a risk of bleeding, especially with the use of certain categories of anticoagulants, which include Warfarin.
The drug greatly affects the number of clotting factors and the number of platelets. It prevents blood clotting by changing the INR level. The risk group includes patients with the following diseases:
- increased intracranial pressure;
- arterial hypertension;
- tendency to strokes and heart attacks.
Patients at risk are recommended to use the drug only in a hospital setting. When using it, blood is constantly donated for a coagulogram to determine the INR. This is inconvenient for the attending physician, so many of them prefer anticoagulants without affecting this indicator.
Answers on questions
How to replace Warfarin for atrial fibrillation
With atrial fibrillation or atrial fibrillation, the risk of blood clots increases; to prevent this phenomenon, Warfarin analogues and substitutes are prescribed - direct or indirect anticoagulants.
When is it better to take Warfarin - morning or evening?
The time of day does not matter when taking Warfarin. It is important to take regularly, at the same time.
Warfarin - direct or indirect anticoagulant
The drug belongs to the group of indirect anticoagulants, since it does not act directly on thrombin, but as a vitamin K antagonist.